Human EGFR Antibody Summary
Leu25-Ser645
Accession # CAA25240
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Human EGFR Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human EGFR by Western Blot. Western blot shows lysates of HeLa human cervical epithelial carcinoma cell line and MDA-MB-231 human breast cancer cell line. PVDF membrane was probed with 1 µg/mL of Goat Anti-Human EGFR Antigen Affinity-purified Polyclonal Antibody (Catalog # AF231) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for EGFR at approximately 175 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of EGFR in A431 Human Cell Line by Flow Cytometry. A431 human epithelial carcinoma cell line was stained with Goat Anti-Human EGFR Antigen Affinity-purified Polyclonal Antibody (Catalog # AF231, filled histogram) or isotype control antibody (Catalog # AB-108-C, open histogram), followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0107). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
EGFR in A431 Human Cell Line. EGFR was detected in immersion fixed A431 human epithelial carcinoma cell line using Goat Anti-Human EGFR Antigen Affinity-purified Polyclonal Antibody (Catalog # AF231) at 1 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to plasma membrane. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
EGFR in Human Skin. EGFR was detected in immersion fixed frozen sections of human skin using Goat Anti-Human EGFR Antigen Affinity-purified Polyclonal Antibody (Catalog # AF231) at 1 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to plasma membrane. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Human EGFR by Simple WesternTM. Simple Western lane view shows lysates of A431 human epithelial carcinoma cell line, loaded at 4.2 mg/mL. A specific band was detected for EGFR at approximately 229 kDa (as indicated) using 12.5 µg/mL of Goat Anti-Human EGFR Antigen Affinity-purified Polyclonal Antibody (Catalog # AF231). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Mouse EGFR by Western Blot Crizotinib combined with mutant-selective EGFR-TKI overcomes multiple resistances to EGFR-TKI invivo.(A) SCID mice-bearing H1975/Vec- or H1975/HGF- tumors were administered WZ4002 (25 mg/kg) and/or crizotinib (10, 25mg/kg) once daily for 6 to 20 days. Tumor volume was measured using calipers on the indicated days. Mean ± SE tumor volumes are shown for groups of 5 mice. *, P < 0.05 versus control; ✝, P < 0.05 versus WZ4002 by one-way ANOVA. (B) H1975/Vec- or H1975/HGF- tumors were resected from the mice 3 hours after administration of WZ4002 (25mg/kg) and/or crizotinib (10, 25 mg/kg), and the relative levels of proteins in the tumor lysates were determined by western blot analysis. (C) Representative images of H1975/Vec- and H1975/HGF- tumors immunohistochemically stained with antibodies to human Ki-67, and stained with both DAPI (nuclear stain) and TUNEL (FITC). Bar, 200 μm. (D) Quantification of proliferative cells, as determined by the Ki-67-positive proliferation index (percentage of Ki-67-positive cells). Quantification of apoptotic cells, as determined by the TUNEL assay as described in Materials and Methods. Columns, mean of five areas; bars, SD *, P < 0.05 versus of H1975/Vec-tumors; ✝, P < 0.05 versus H1975/HGF-tumors by one-way ANOVA. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0084700), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human EGFR by Western Blot Crizotinib reduces Met phosphorylation and combined treatment with a new generation EGFR-TKI inhibits downstream pathways even in the presence of HGF.H1975 and H1975/HGF cells were incubated with crizotinib (300 nmol/L) and/or afatinib (300 nmol/L) (A, B) or WZ4002 (300 nmol/L) (C, D), for 1 hour. After stimulation with HGF (10 ng/mL) for 10 minutes, the cell lysates were harvested and the phosphorylation of indicated proteins was determined by western blot analysis. Each sample was assayed in triplicate, with each experiment repeated at least 3 times independently. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0084700), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human EGFR by Western Blot Crizotinib reduces Met phosphorylation and combined treatment with a new generation EGFR-TKI inhibits downstream pathways even in the presence of HGF.H1975 and H1975/HGF cells were incubated with crizotinib (300 nmol/L) and/or afatinib (300 nmol/L) (A, B) or WZ4002 (300 nmol/L) (C, D), for 1 hour. After stimulation with HGF (10 ng/mL) for 10 minutes, the cell lysates were harvested and the phosphorylation of indicated proteins was determined by western blot analysis. Each sample was assayed in triplicate, with each experiment repeated at least 3 times independently. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0084700), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human EGFR by Western Blot Crizotinib reduces Met phosphorylation and combined treatment with a new generation EGFR-TKI inhibits downstream pathways even in the presence of HGF.H1975 and H1975/HGF cells were incubated with crizotinib (300 nmol/L) and/or afatinib (300 nmol/L) (A, B) or WZ4002 (300 nmol/L) (C, D), for 1 hour. After stimulation with HGF (10 ng/mL) for 10 minutes, the cell lysates were harvested and the phosphorylation of indicated proteins was determined by western blot analysis. Each sample was assayed in triplicate, with each experiment repeated at least 3 times independently. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0084700), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human EGFR by Western Blot Crizotinib reduces Met phosphorylation and combined treatment with a new generation EGFR-TKI inhibits downstream pathways even in the presence of HGF.H1975 and H1975/HGF cells were incubated with crizotinib (300 nmol/L) and/or afatinib (300 nmol/L) (A, B) or WZ4002 (300 nmol/L) (C, D), for 1 hour. After stimulation with HGF (10 ng/mL) for 10 minutes, the cell lysates were harvested and the phosphorylation of indicated proteins was determined by western blot analysis. Each sample was assayed in triplicate, with each experiment repeated at least 3 times independently. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0084700), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse EGFR by Western Blot Crizotinib combined with irreversible EGFR-TKI overcomes multiple resistances to EGFR-TKI in vivo.(A) SCID mice-bearing H1975/Vec- or H1975/HGF- tumors were administered afatinib (25 mg/kg) and/or crizotinib (10mg/kg) once daily for 6 to 20 days. Tumor volume was measured using calipers on the indicated days. Mean ± SE tumor volumes are shown for groups of 5 mice. *, P < 0.05 versus control; ✝, P < 0.05 versus afatinib (25 mg/kg) by one-way ANOVA. (B) H1975/Vec- or H1975/HGF- tumors were resected from the mice 3 hours after administration of afatinib (25mg/kg) and/or crizotinib (10 mg/kg), and the relative levels of proteins in the tumor lysates were determined by western blot analysis. (C) Representative images of H1975/Vec- and H1975/HGF tumors immunohistochemically stained with antibodies to human Ki-67, and stained with both DAPI (nuclear stain) and TUNEL (FITC). Bar, 200 μm. (D) Quantification of proliferative cells, as determined by their Ki-67-positive proliferation index (percentage of Ki-67-positive cells). Quantification of apoptotic cells, as determined by the TUNEL assay as described in Materials and Methods. Columns, mean of five areas; bars, SD. *, P < 0.05 versus H1975/Vec-tumors; ✝, P < 0.05 versus control of H1975/HGF-tumors by one-way ANOVA. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0084700), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of EGFR in Human Skin. Formalin-fixed paraffin-embedded tissue sections of human skin were probed for EGFR mRNA (ACD RNAScope Probe, catalog # 310061; Fast Red chromogen, ACD catalog # 322360). Adjacent tissue section was processed for immunohistochemistry using goat anti-human EGFR polyclonal antibody (R&D Systems catalog # AF231) at 3ug/mL with overnight incubation at 4 degrees Celsius followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog # VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to keratinocytes.
 View Larger
View Larger
Detection of Human Human EGFR Antibody by Western Blot Crizotinib reduces Met phosphorylation and combined treatment with a new generation EGFR-TKI inhibits downstream pathways even in the presence of HGF.H1975 and H1975/HGF cells were incubated with crizotinib (300 nmol/L) and/or afatinib (300 nmol/L) (A, B) or WZ4002 (300 nmol/L) (C, D), for 1 hour. After stimulation with HGF (10 ng/mL) for 10 minutes, the cell lysates were harvested and the phosphorylation of indicated proteins was determined by western blot analysis. Each sample was assayed in triplicate, with each experiment repeated at least 3 times independently. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24386407), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human EGFR Antibody by Western Blot Crizotinib combined with mutant-selective EGFR-TKI overcomes multiple resistances to EGFR-TKI invivo.(A) SCID mice-bearing H1975/Vec- or H1975/HGF- tumors were administered WZ4002 (25 mg/kg) and/or crizotinib (10, 25mg/kg) once daily for 6 to 20 days. Tumor volume was measured using calipers on the indicated days. Mean ± SE tumor volumes are shown for groups of 5 mice. *, P < 0.05 versus control; ✝, P < 0.05 versus WZ4002 by one-way ANOVA. (B) H1975/Vec- or H1975/HGF- tumors were resected from the mice 3 hours after administration of WZ4002 (25mg/kg) and/or crizotinib (10, 25 mg/kg), and the relative levels of proteins in the tumor lysates were determined by western blot analysis. (C) Representative images of H1975/Vec- and H1975/HGF- tumors immunohistochemically stained with antibodies to human Ki-67, and stained with both DAPI (nuclear stain) and TUNEL (FITC). Bar, 200 μm. (D) Quantification of proliferative cells, as determined by the Ki-67-positive proliferation index (percentage of Ki-67-positive cells). Quantification of apoptotic cells, as determined by the TUNEL assay as described in Materials and Methods. Columns, mean of five areas; bars, SD *, P < 0.05 versus of H1975/Vec-tumors; ✝, P < 0.05 versus H1975/HGF-tumors by one-way ANOVA. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24386407), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Human EGFR Antibody by Western Blot Crizotinib reduces Met phosphorylation and combined treatment with a new generation EGFR-TKI inhibits downstream pathways even in the presence of HGF.H1975 and H1975/HGF cells were incubated with crizotinib (300 nmol/L) and/or afatinib (300 nmol/L) (A, B) or WZ4002 (300 nmol/L) (C, D), for 1 hour. After stimulation with HGF (10 ng/mL) for 10 minutes, the cell lysates were harvested and the phosphorylation of indicated proteins was determined by western blot analysis. Each sample was assayed in triplicate, with each experiment repeated at least 3 times independently. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24386407), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
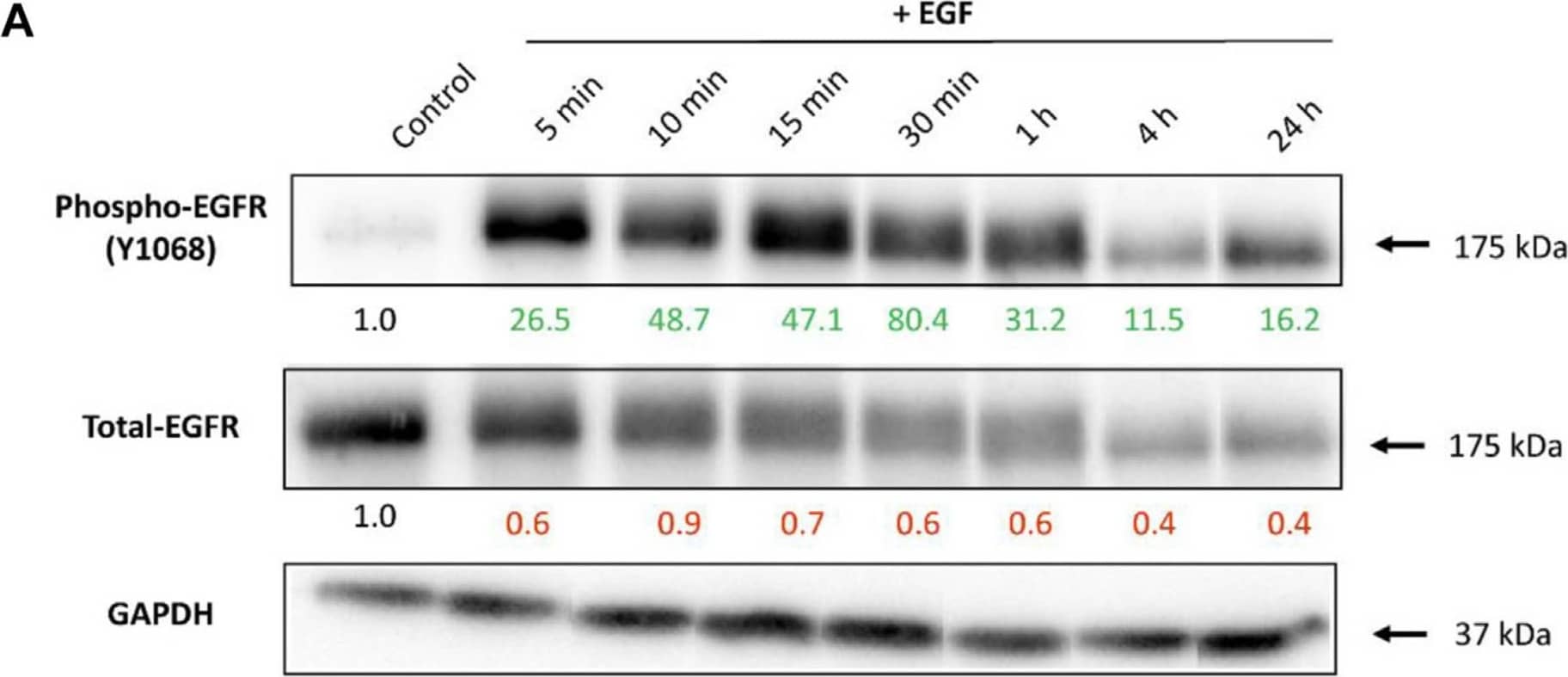
Detection of EGFR by Western Blot The EGFR expression upon cell stimulation with EGF.(A) Representative Western blot images showing expression of phosphorylated- and total-EGFR (Phospho-EGFR and Total-EGFR), upon EGF stimulation at different time points. GAPDH served as an internal control for protein loading. The average expression values of the indicated protein were determined via densitometry (Fiji software) from three independent experiments and presented as a fold change over the negative controls. Increased expression is marked in green and decreased in red. Full Western blot images including the corresponding controls for each time point and weight marker are shown in Supplementary Figure S2. (B) Localization of EGFR upon cell stimulation with EGF assessed via confocal microscopy. Receptor internalization was observed 5 min after A549 stimulation with EGF, followed by receptor recycling back to the membrane staring at 15 min. Magenta: EGFR;Cyan: nuclei. Scale bar: 10 μm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37954478), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: EGFR
The epidermal growth factor receptor (EGFR) subfamily of receptor tyrosine kinases comprises four members: EGFR (also known as HER1, ErbB1 or ErbB), ErbB2 (Neu, HER2), ErbB3 (HER3), and ErbB4 (HER4). All family members are type I transmembrane glycoproteins that have an extracellular domain which contains two cysteine-rich domains separated by a spacer region that is involved in ligand binding, and a cytoplasmic domain which has a membrane-proximal tyrosine kinase domain and a C-terminal tail with multiple tyrosine autophosphorylation sites. The human EGFR gene encodes a 1210 amino acid (aa) residue precursor with a 24 aa putative signal peptide, a 621 aa extracellular domain, a 23 aa transmembrane domain, and a 542 aa cytoplasmic domain. EGFR has been shown to bind a subset of the EGF family ligands, including EGF, amphiregulin, TGF-alpha, betacellulin, epiregulin, heparin-binding EGF and neuregulin-2 alpha in the absence of a co-receptor. Ligand binding induces EGFR homodimerization as well as heterodimerization with ErbB2, resulting in kinase activation, tyrosine phosphorylation and cell signaling. EGFR can also be recruited to form heterodimers with the ligand-activated ErbB3 or ErbB4. EGFR signaling has been shown to regulate multiple biological functions including cell proliferation, differentiation, motility and apoptosis. In addition, EGFR signaling has also been shown to play a role in carcinogenesis (1 - 3).
- Daly, R.J. (1999) Growth Factors, 16:255.
- Schlessinger, J. (2000) Cell. 103:211.
- Maihle, N.J. et al. (2002) Cancer Treat. Res. 107:247.
Product Datasheets
Citations for Human EGFR Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
32
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Soluble fms-Like Tyrosine Kinase 1 Localization in Renal Biopsies of CKD
Authors: ZK Zsengellér, A Lo, M Tavasoli, E Pernicone, SA Karumanchi, S Rosen
Kidney Int Rep, 2019-08-14;4(12):1735-1741.
-
HER1 (EGFR) and/or HER2 inclusion potentiates the antitumor effect elicited by a HER3-specific monovalent vaccine
Authors: Bermúdez-Abreut, E;Bergado Báez, G;Fundora-Barrios, T;Arencibia-Perezleo, J;Medinilla, AL;Chao, L;Sánchez Ramírez, B;
Molecular cancer therapeutics
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Lateral Ventricular Neural Stem Cells Provide Negative Feedback to Circuit Activation Through GABAergic Signaling
Authors: Naffaa, MM;Yin, HH;
Cells
Species: Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
The EGFR phosphatase RPTP gamma is a redox‐regulated suppressor of promigratory signaling
Authors: Maitreyi S Joshi, Angel Stanoev, Jan Huebinger, Birga Soetje, Veronika Zorina, Lisaweta Ro beta mannek et al.
The EMBO Journal
-
An atlas of late prenatal human neurodevelopment resolved by single-nucleus transcriptomics
Authors: SI Ramos, ZM Mussa, EN Falk, B Pai, B Giotti, K Allette, P Cai, F Dekio, R Sebra, KG Beaumont, AM Tsankov, NM Tsankova
Nature Communications, 2022-12-12;13(1):7671.
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Integrated Analytical System for Clinical Single‐Cell Analysis
Authors: Hannah M. Peterson, Lip Ket Chin, Yoshi Iwamoto, Juhyun Oh, Jonathan C. T. Carlson, Hakho Lee et al.
Advanced Science
-
Plasma membrane proteoglycans syndecan-2 and syndecan-4 engage with EGFR and RON kinase to sustain carcinoma cell cycle progression
Authors: DM Beauvais, SE Nelson, KM Adams, NA Stueven, O Jung, AC Rapraeger
The Journal of Biological Chemistry, 2022-05-13;0(0):102029.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation, Western Blot -
Glioblastoma mutations alter EGFR dimer structure to prevent ligand bias
Authors: C Hu, CA Leche, A Kiyatkin, Z Yu, SE Stayrook, KM Ferguson, MA Lemmon
Nature, 2022-02-09;602(7897):518-522.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Developmental Origins of Human Cortical Oligodendrocytes and Astrocytes
Authors: Lin Yang, Zhenmeiyu Li, Guoping Liu, Xiaosu Li, Zhengang Yang
Neuroscience Bulletin
-
EGFR transactivates RON to drive oncogenic crosstalk
Authors: Carolina Franco Nitta, Ellen W Green, Elton D Jhamba, Justine M Keth, Iraís Ortiz-Caraveo, Rachel M Grattan et al.
eLife
-
Evaluation of the Targeting and Therapeutic Efficiency of Anti-EGFR Functionalised Nanoparticles in Head and Neck Cancer Cells for Use in NIR-II Optical Window
Authors: T Egnuni, N Ingram, I Mirza, PL Coletta, JR McLaughlan
Pharmaceutics, 2021-10-09;13(10):.
Species: Human
Sample Types: Whole Cell
Applications: IF -
Bacterial Antigens Reduced the Inhibition Effect of Capsaicin on Cal 27 Oral Cancer Cell Proliferation
Authors: R Chakrabort, K Vickery, C Darido, S Ranganatha, H Hu
International Journal of Molecular Sciences, 2021-08-12;22(16):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Involvement of cancer-derived EMT cells in the accumulation of 18F-fluorodeoxyglucose in the hypoxic cancer microenvironment
Authors: S Sugita, M Yamato, T Hatabu, Y Kataoka
Scientific Reports, 2021-05-17;11(1):9668.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
EGFR inhibition blocks cancer stem cell clustering and lung metastasis of triple negative breast cancer
Authors: X Liu, V Adorno-Cru, YF Chang, Y Jia, M Kawaguchi, NK Dashzeveg, R Taftaf, EK Ramos, EJ Schuster, L El-Shennaw, D Patel, Y Zhang, M Cristofani, H Liu
Theranostics, 2021-04-30;11(13):6632-6643.
Species: Human
Sample Types: Cell Lysates
Applications: Co-Immunoprecipitation -
Development of an immuno-wall device for the rapid and sensitive detection of EGFR mutations in tumor tissues resected from lung cancer patients
Authors: N Yogo, T Hase, T Kasama, K Nishiyama, N Ozawa, T Hatta, H Shibata, M Sato, K Komeda, N Kawabe, K Matsuoka, TF Chen-Yoshi, N Kaji, M Tokeshi, Y Baba, Y Hasegawa
PLoS ONE, 2020-11-16;15(11):e0241422.
Species: Human
Sample Types: Cell Lysates
Applications: ELISA Detection -
Systems Modeling Identifies Divergent Receptor Tyrosine Kinase Reprogramming to MAPK Pathway Inhibition
Authors: Allison M. Claas, Lyla Atta, Simon Gordonov, Aaron S. Meyer, Douglas A. Lauffenburger
Cellular and Molecular Bioengineering
-
Interdependence between EGFR and Phosphatases Spatially Established by Vesicular Dynamics Generates a Growth Factor Sensing and Responding Network
Authors: Angel Stanoev, Amit Mhamane, Klaus C. Schuermann, Hernán E. Grecco, Wayne Stallaert, Martin Baumdick et al.
Cell Systems
-
A conformational sensor based on genetic code expansion reveals an autocatalytic component in EGFR activation
Authors: Martin Baumdick, Márton Gelléri, Chayasith Uttamapinant, Václav Beránek, Jason W. Chin, Philippe I. H. Bastiaens
Nature Communications
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Identification of a novel anoikis signalling pathway using the fungal virulence factor gliotoxin
Authors: F Haun, S Neumann, L Peintner, K Wieland, J Habicht, C Schwan, K Østevold, MM Koczorowsk, M Biniossek, M Kist, H Busch, M Boerries, RJ Davis, U Maurer, O Schilling, K Aktories, C Borner
Nat Commun, 2018-08-30;9(1):3524.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Improved efficiency of in situ protein analysis by proximity ligation using UnFold probes
Authors: A Klaesson, K Grannas, T Ebai, J Heldin, B Koos, M Leino, D Raykova, J Oelrich, L Arngården, O Söderberg, U Landegren
Sci Rep, 2018-03-29;8(1):5400.
Species: Human
Sample Types: Whole Cells
Applications: Proximity Ligation Assay, Immunocytochemistry -
Cerebellar Granule Cell Replenishment Post-Injury by Adaptive Reprogramming of Nestin+ Progenitors
Authors: Alexandre Wojcinski, Andrew K. Lawton, N Sumru. Bayin, Zhimin Lao, Daniel N. Stephen, Alexandra L. Joyner
Nature Neuroscience
-
Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia
Authors: Suzanne D Burke
J Clin Invest, 2016-06-06;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
EGF-dependent re-routing of vesicular recycling switches spontaneous phosphorylation suppression to EGFR signaling
Authors: Martin Baumdick, Yannick Brüggemann, Malte Schmick, Georgia Xouri, Ola Sabet, Lloyd Davis et al.
eLife
-
Aptamer targeting EGFRvIII mutant hampers its constitutive autophosphorylation and affects migration, invasion and proliferation of glioblastoma cells
Authors: Simona Camorani, Elvira Crescenzi, David Colecchia, Andrea Carpentieri, Angela Amoresano, Monica Fedele et al.
Oncotarget
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Multichannel Imaging to Quantify Four Classes of Pharmacokinetic Distribution in Tumors
Authors: Sumit Bhatnagar, Emily Deschenes, Jianshan Liao, Cornelius Cilliers, Greg M. Thurber
Journal of Pharmaceutical Sciences
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips†
Authors: Christopher M. Earhart, Casey E. Hughes, Richard S. Gaster, Chin Chun Ooi, Robert J. Wilson, Lisa Y. Zhou et al.
Lab Chip
-
Ability of the Met kinase inhibitor crizotinib and new generation EGFR inhibitors to overcome resistance to EGFR inhibitors.
Authors: Nanjo, Shigeki, Yamada, Tadaaki, Nishihara, Hiroshi, Takeuchi, Shinji, Sano, Takako, Nakagawa, Takayuki, Ishikawa, Daisuke, Zhao, Lu, Ebi, Hiromich, Yasumoto, Kazuo, Matsumoto, Kunio, Yano, Seiji
PLoS ONE, 2013-12-26;8(12):e84700.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Human epidermal growth factor receptor (HER-1:HER-3) Fc-mediated heterodimer has broad antiproliferative activity in vitro and in human tumor xenografts.
Authors: Sarup J, Jin P, Turin L, Bai X, Beryt M, Brdlik C, Higaki JN, Jorgensen B, Lau FW, Lindley P, Liu J, Ni I, Rozzelle J, Kumari R, Watson SA, Zhang J, Shepard HM
Mol. Cancer Ther., 2008-10-01;7(10):3223-36.
Species: Human
Sample Types: Cell Lysates, Recombinant Protein
Applications: ELISA Development, Western Blot -
Development and validation of sandwich ELISA microarrays with minimal assay interference.
Authors: Gonzalez RM, Seurynck-Servoss SL, Crowley SA
J. Proteome Res., 2008-04-19;7(6):2406-14.
Species: Human
Sample Types: Serum
Applications: ELISA Microarray Development -
FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival.
Authors: Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, Elbi C, Lutterbach B
Cancer Res., 2008-04-01;68(7):2340-8.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation, Western Blot -
Expression of growth factors and growth factor receptor in non-healing and healing ischaemic ulceration.
Authors: Murphy MO, Ghosh J, Fulford P, Khwaja N, Halka AT, Carter A, Turner NJ, Walker MG
Eur J Vasc Endovasc Surg, 2006-01-20;31(5):516-22.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles
Authors: E Reátegui, KE van der Vo, CP Lai, M Zeinali, NA Atai, B Aldikacti, FP Floyd, A H Khankhel, V Thapar, FH Hochberg, LV Sequist, BV Nahed, B S Carter, M Toner, L Balaj, D T Ting, XO Breakefiel, SL Stott
Nat Commun, 2018-01-12;9(1):175.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human EGFR Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Human EGFR Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:





