Human TIMP-1 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human TIMP-1. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
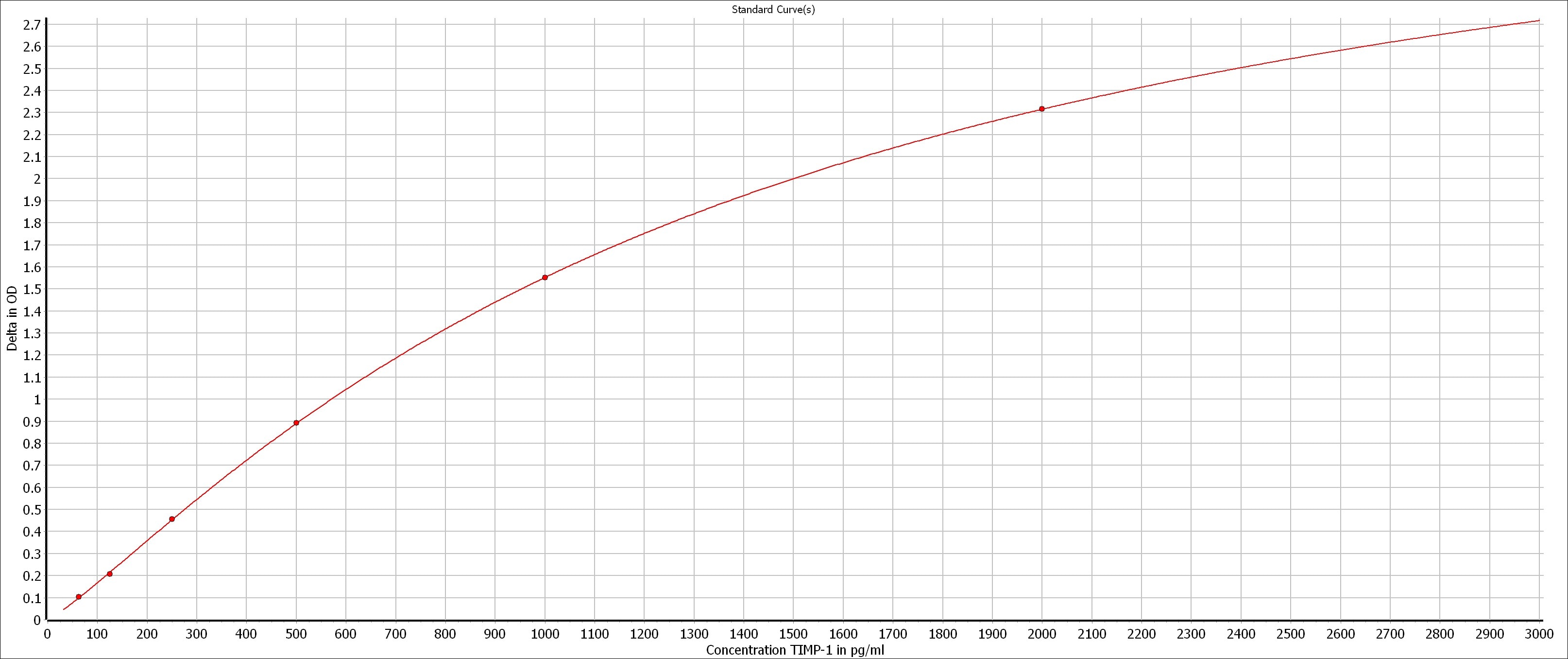
Scientific Data
Product Datasheets
Preparation and Storage
Background: TIMP-1
TIMPs-1 through -4 regulate the activity of zinc metalloproteases known as MMPs, ADAMs and ADAMTSs. Structurally, TIMPs contain two domains. The N-terminal domain binds to the active site of mature metalloproteases via a 1:1 non-covalent interaction, blocking access of substrates to the catalytic site. In addition, The C-terminal domain of TIMP-1 and TIMP-2 binds to the hemopexin- like domain of pro-MMP-9 and pro-MMP-2, respectively. The latter binding is essential for the cell surface activation of MMP-2 by MMP-14.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human TIMP-1 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
47
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
MMP-9 and TIMPs profiles in sulfur mustard-exposed individuals with serious lung complications
Authors: Fallahi, F;Askari, N;Jamali, T;Parsapour, S;Ghasemi, H;Shams, J;Yaraee, R;Ghazanfari, Z;Ghazanfari, T;
International immunopharmacology
Species: Human
Sample Types: Serum, Sputum
-
Estrogen-dependent gene regulation: Molecular basis of TIMP-1 as a sex-specific biomarker for acute lung injury
Authors: Almuntashiri, S;Dutta, S;Zhu, Y;Gamare, S;Ramírez, G;Irineo-Moreno, V;Camarena, A;Regino, N;Campero, P;Hernández-Cardenas, CM;Rodriguez-Reyna, TS;Zuñiga, J;Owen, CA;Wang, X;Zhang, D;
Physiological reports
Species: Human
Sample Types: Plasma
-
Myocardial transcriptomic analysis of diabetic patients with aortic stenosis: key role for mitochondrial calcium signaling
Authors: Cherpaz, M;Meugnier, E;Seillier, G;Pozzi, M;Pierrard, R;Leboube, S;Farhat, F;Vola, M;Obadia, JF;Amaz, C;Chalabreysse, L;May, C;Chanon, S;Brun, C;Givre, L;Bidaux, G;Mewton, N;Derumeaux, G;Bergerot, C;Paillard, M;Thibault, H;
Cardiovascular diabetology
Species: Human
Sample Types: Serum
-
Tofacitinib Regulates Endostatin via Effects on CD147 and Cathepsin S
Authors: Zisman, D;Sabtan, H;Rahat, MM;Simanovich, E;Haddad, A;Gazitt, T;Feld, J;Slobodin, G;Kibari, A;Elias, M;Rahat, MA;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Defining the mechanism of galectin-3-mediated TGF-?1 activation and its role in lung fibrosis
Authors: Calver, J;Parmar, N;Harris, G;Lithgo, R;Stylianou, P;Zetterberg, F;Gooptu, B;MacKinnon, A;Carr, S;Borthwick, L;Scott, D;Stewart, I;Slack, R;Jenkins, G;John, A;
Journal of Biological Chemistry
Species: Human
Sample Types: Whole Tissue
-
Implications of the Matrix Metalloproteinases, Their Tissue Inhibitors and Some Other Inflammatory Mediators Expression Levels in Children Obesity-Related Phenotypes
Authors: Wierzbicka-Ruci?ska, A;Kubiszewska, I;Grzywa-Czuba, R;Gackowska, L;Szalecki, M;Micha?kiewicz, J;Trojanek, JB;
Journal of personalized medicine
Species: Human
Sample Types: Cell Culture Supernates, Plasma
-
Serum TGF-?1 and CD14 Predicts Response to Anti-TNF-? Therapy in IBD
Authors: Coufal, S;Kverka, M;Kreisinger, J;Thon, T;Rob, F;Kolar, M;Reiss, Z;Schierova, D;Kostovcikova, K;Roubalova, R;Bajer, L;Jackova, Z;Mihula, M;Drastich, P;Tresnak Hercogova, J;Novakova, M;Vasatko, M;Lukas, M;Tlaskalova-Hogenova, H;Jiraskova Zakostelska, Z;
Journal of immunology research
Species: Human
Sample Types: Serum
-
Intrathecal Administration of Conditioned Serum from Different Species Resolves Chemotherapy-Induced Neuropathic Pain in Mice via Secretory Exosomes
Authors: Buchheit, T;Huh, Y;Breglio, A;Bang, S;Xu, J;Matsuoka, Y;Guo, R;Bortsov, A;Reinecke, J;Wehling, P;Jun Huang, T;Ji, RR;
Brain, behavior, and immunity
Species: Human
Sample Types: Serum
-
IL-17A in Human Liver: Significant Source of Inflammation and Trigger of Liver Fibrosis Initiation.
Authors: Kartasheva-Ebertz D, Gaston J, Lair-Mehiri L, Mottez E, Buivan T, Massault P, Scatton O, Gaujoux S, Vaillant J, Pol S, Lagaye S
Int J Mol Sci, 2022-08-29;23(17):.
Species: Human
Sample Types: Tissue Culture Supernates
-
Association between Circulating Levels of 25-Hydroxyvitamin D3 and Matrix Metalloproteinase-10 (MMP-10) in Patients with Type 2 Diabetes
Authors: D Abasheva, MM Dolcet-Neg, MA Fernández-, JM Mora-Gutié, J Orbe, FJ Escalada, N Garcia-Fer
Nutrients, 2022-08-24;14(17):.
Species: Human
Sample Types: Serum
-
Pro-cancerogenic effects of spontaneous and drug-induced senescence of ovarian cancer cells in vitro and in vivo: a comparative analysis
Authors: S Rutecki, P Szulc, M Paku?a, P Uruski, A Radziemski, E Naumowicz, R Moszy?ski, A Tykarski, J Miku?a-Pie, K Ksi??ek
Journal of ovarian research, 2022-07-26;15(1):87.
Species: Human
Sample Types: Cell Culture Supernates
-
Mg-based materials diminish tumor spreading and cancer metastases
Authors: P Globig, R Madurawala, R Willumeit-, F Martini, E Mazzoni, BJC Luthringer
Bioactive materials, 2022-05-10;19(0):594-610.
Species: Human
Sample Types: Cell Culture Supernates
-
Evaluation in a Cytokine Storm Model In Vivo of the Safety and Efficacy of Intravenous Administration of PRS CK STORM (Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells)
Authors: JP Lapuente, G Gómez, J Marco-Brua, P Fernández, P Desportes, J Sanz, M García-Gil, F Bermejo, JV San Martín, A Algaba, JC De Gregori, D Lapuente, A De Gregori, B Lapuente, S Gómez, MLV Andrés, A Anel
Biomedicines, 2022-05-08;10(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Atypical response to bacterial co-infection and persistent neutrophilic broncho-alveolar inflammation distinguish critical COVID-19 from influenza
Authors: S Cambier, M Metzemaeke, AC Carvalho, A Nooyens, C Jacobs, L Vanderbeke, B Malengier-, M Gouwy, E Heylen, P Meersseman, G Hermans, E Wauters, A Wilmer, C Consortium, D Schols, P Matthys, G Opdenakker, RE Marques, J Wauters, J Vandooren, P Proost
JCI Insight, 2022-01-11;0(0):.
Species: Human
Sample Types: BAL Supernatant
-
TIMP1 expression underlies sex disparity in liver metastasis and survival in pancreatic cancer
Authors: Chris D. Hermann, Benjamin Schoeps, Celina Eckfeld, Enkhtsetseg Munkhbaatar, Lukas Kniep, Olga Prokopchuk et al.
Journal of Experimental Medicine
Species: Human
Sample Types: Plasma
-
MMPs and TIMPs levels are correlated with anthropometric parameters, blood pressure, and endothelial function in obesity
Authors: S Boumiza, K Chahed, Z Tabka, MP Jacob, X Norel, G Ozen
Scientific Reports, 2021-10-08;11(1):20052.
Species: Human
Sample Types: Plasma
-
Differential inflammation-mediated function of prokineticin 2 in the synovial fibroblasts of patients with rheumatoid arthritis compared with osteoarthritis
Authors: K Noda, B Dufner, H Ito, K Yoshida, G Balboni, RH Straub
Scientific Reports, 2021-09-15;11(1):18399.
Species: Human
Sample Types: Cell Culture Supernates
-
Antibody microarray analysis of the amniotic fluid proteome�for predicting the outcome of rescue cerclage in patients with cervical insufficiency
Authors: S Hong, KH Park, YE Lee, S Shin, HJ Kim, YM Kim
Bioscience Reports, 2021-07-30;41(7):.
Species: Human
Sample Types: Amniotic Fluid
-
Nobiletin Decreases Inflammatory Mediator Expression in Tumor Necrosis Factor-Stimulated Human Periodontal Ligament Cells
Authors: Y Hosokawa, I Hosokawa, K Ozaki
Mediators of Inflammation, 2021-07-10;2021(0):5535844.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum protein triplet TGF-&beta1, TIMP-1, and YKL-40 serve as diagnostic and prognostic profile for astrocytoma
Authors: R Urbanavi?i, R Zabitait?, A Kriš?iukai, VP Deltuva, D Skiriut?
Scientific Reports, 2021-06-23;11(1):13100.
Species: Human
Sample Types: Serum
-
A combined biomarker approach for characterising extracellular matrix profiles in acute myocardial infarction
Authors: MM Brunton-O', AS Holley, KE Hally, GA Kristono, SA Harding, PD Larsen
Scientific Reports, 2021-06-16;11(1):12705.
Species: Human
Sample Types: Plasma
-
Potential novel biomarkers for chronic lung allograft dysfunction and azithromycin responsive allograft dysfunction
Authors: C Veraar, J Kliman, A Benazzo, F Oberndorfe, M Laggner, P Hacker, T Raunegger, S Janik, P Jaksch, W Klepetko, HJ Ankersmit, B Moser
Scientific Reports, 2021-03-24;11(1):6799.
Species: Human
Sample Types: Serum
-
The Polymethoxy Flavonoid Sudachitin Inhibits Interleukin-1&beta-Induced Inflammatory Mediator Production in Human Periodontal Ligament Cells
Authors: Y Hosokawa, I Hosokawa, K Ozaki, T Matsuo
BioMed Research International, 2021-01-28;2021(0):8826586.
Species: Human
Sample Types: Cell Culture Supernates
-
Exploring the potential effect of paricalcitol on markers of inflammation in de novo renal transplant recipients
Authors: HK Pihlstrøm, T Ueland, AE Michelsen, P Aukrust, F Gatti, C Hammarströ, M Kasprzycka, J Wang, G Haraldsen, G Mjøen, DO Dahle, K Midtvedt, IA Eide, A Hartmann, H Holdaas
PLoS ONE, 2020-12-16;15(12):e0243759.
Species: Human
Sample Types: Plasma
-
Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Peripheral Blood Leukocytes and Plasma of Children with Nonalcoholic Fatty Liver Disease
Authors: JB Trojanek, J Micha?kiew, R Grzywa-Czu, W Ja?czyk, L Gackowska, I Kubiszewsk, A Helmin-Bas, A Wierzbicka, M Szalecki, P Socha
Mediators Inflamm., 2020-09-10;2020(0):8327945.
Species: Human
Sample Types: Plasma
-
Matrix metalloproteinase-7 could be a predictor for acute inflammation in psoriatic patients
Authors: A Abbes, Y Zayani, W Zidi, MB Hammami, A Mebazaa, D El Euch, A Ben Ammar, H Sanhaji, MV El May, M Mokni, M Feki, M Allal-Elas
Cytokine, 2020-07-11;134(0):155195.
Species: Human
Sample Types: Plasma
-
Evaluation of the post-treatment anti-inflammatory capacity of osteoarthritic chondrocytes: An in�vitro study using baicalein
Authors: CC Wu, YR Chen, DH Lu, LH Hsu, KC Yang, S Sumi
Regen Ther, 2020-02-24;14(0):177-183.
Species: Human
Sample Types: Cell Culture Supernates
-
MMP-10 is Increased in Early Stage Diabetic Kidney Disease and can be Reduced by Renin-Angiotensin System Blockade
Authors: JM Mora-Gutié, JA Rodríguez, MA Fernández-, J Orbe, FJ Escalada, MJ Soler, MF Slon Roble, M Riera, JA Páramo, N Garcia-Fer
Sci Rep, 2020-01-08;10(1):26.
Species: Human
Sample Types: Serum
-
Immune and Inflammatory Proteins in Cord Blood as Predictive Biomarkers of Retinopathy of Prematurity in Preterm Infants
Authors: YJ Park, SJ Woo, YM Kim, S Hong, YE Lee, KH Park
Invest. Ophthalmol. Vis. Sci., 2019-09-03;60(12):3813-3820.
Species: Human
Sample Types: Plasma
-
Inflammatory Bowel Disease Types Differ in Markers of Inflammation, Gut Barrier and in Specific Anti-Bacterial Response
Authors: S Coufal, N Galanova, L Bajer, Z Gajdarova, D Schierova, Z Jiraskova, K Kostovciko, Z Jackova, Z Stehlikova, P Drastich, H Tlaskalova, M Kverka
Cells, 2019-07-13;8(7):.
Species: Human
Sample Types: Serum
-
Proteolytic biomarkers are related to prognosis in COPD- report from a population-based cohort
Authors: R Linder, E Rönmark, J Pourazar, AF Behndig, A Blomberg, A Lindberg
Respir. Res., 2018-04-12;19(1):64.
Species: Human
Sample Types: Serum
-
Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer
Authors: O Prokopchuk, B Grünwald, U Nitsche, C Jäger, OL Prokopchuk, EC Schubert, H Friess, ME Martignoni, A Krüger
BMC Cancer, 2018-02-02;18(1):128.
Species: Human
Sample Types: Plasma
-
Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis
Authors: S Brilha, CWM Ong, B Weksler, N Romero, PO Couraud, JS Friedland
Sci Rep, 2017-11-22;7(1):16031.
Species: Human
Sample Types: Cell Culture Supernates
-
MMP-1 Activation Contributes to Airway Smooth Muscle Growth and Asthma Severity
Authors: Shams-Un-Nisa Naveed
Am. J. Respir. Crit. Care Med, 2017-04-15;0(0):.
Species: Human
Sample Types: BALF
-
Complex regulation of neutrophil-derived MMP-9 secretion in central nervous system tuberculosis
Authors: CW Ong, PJ Pabisiak, S Brilha, P Singh, F Roncaroli, PT Elkington, JS Friedland
J Neuroinflammation, 2017-02-07;14(1):31.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of Matrix Metalloproteinases -7, -8 and -9 and TIMP -1 with Disease Severity in Acute Pancreatitis. A Cohort Study
PLoS ONE, 2016-08-25;11(8):e0161480.
Species: Human
Sample Types: Plasma
-
Serum metalloproteinase-9 is related to COPD severity and symptoms - cross-sectional data from a population based cohort-study.
Authors: Linder R, Ronmark E, Pourazar J, Behndig A, Blomberg A, Lindberg A
Respir Res, 2015-02-21;16(0):28.
Species: Human
Sample Types: Serum
-
Identification of neutrophil activation markers as novel surrogate markers of CF lung disease.
Authors: Rath T, Zwaschka L, Hage L, Kugler M, Menendez K, Naehrlich L, Schulz R, Roderfeld M, Roeb E
PLoS ONE, 2014-12-29;9(12):e115847.
Species: Human
Sample Types: Serum
-
Compartment differences of inflammatory activity in chronic obstructive pulmonary disease.
Authors: Ji J, von Scheele I, Bergstrom J, Billing B, Dahlen B, Lantz A, Larsson K, Palmberg L
Respir Res, 2014-08-26;15(0):104.
Species: Human
Sample Types: Serum
-
Decreased PGE(2) content reduces MMP-1 activity and consequently increases collagen density in human varicose vein.
Authors: Gomez I, Benyahia C, Louedec L, Leseche G, Jacob M, Longrois D, Norel X
PLoS ONE, 2014-02-05;9(2):e88021.
Species: Human
Sample Types: Tissue Culture Supernates
-
Expression of matrix metalloproteinases and their tissue inhibitors in the serum and cerebrospinal fluid of patients with HIV-1 infection and syphilis or neurosyphilis.
Authors: Tsai HC, Ye SY, Kunin CM, Lee SS, Wann SR, Tai MH, Shi MH, Liu YC, Chen YS
Cytokine, 2011-02-26;54(0):109-16.
Species: Human
Sample Types: CSF
-
STAT3, p38 MAPK, and NF-kappaB drive unopposed monocyte-dependent fibroblast MMP-1 secretion in tuberculosis.
Authors: O'Kane CM, Elkington PT, Jones MD, Caviedes L, Tovar M, Gilman RH, Stamp G, Friedland JS
Am. J. Respir. Cell Mol. Biol., 2009-11-13;43(0):465.
Species: Human
Sample Types: Cell Culture Supernates
-
Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers.
Authors: Shyamsundar M, McKeown ST, O'Kane CM, O'Kane CM, O'Kane CM, O'Kane CM, O'Kane CM, Craig TR, Brown V, Thickett DR, Matthay MA, Taggart CC, Backman JT, Elborn JS, McAuley DF
Am. J. Respir. Crit. Care Med., 2009-03-26;179(12):1107-14.
Species: Human
Sample Types: BALF
-
Matrix metalloproteinases and their tissue inhibitors (TIMPs) in Plasmodium falciparum malaria: serum levels of TIMP-1 are associated with disease severity.
Authors: Dietmann A, Helbok R, Lackner P, Issifou S, Lell B, Matsiegui PB, Reindl M, Schmutzhard E, Kremsner PG
J. Infect. Dis., 2008-06-01;197(11):1614-20.
Species: Human
Sample Types: Serum
-
Association of matrix metalloproteinase-9 and tissue inhibitors of metalloproteinase-4 in cerebrospinal fluid with blood-brain barrier dysfunction in patients with eosinophilic meningitis caused by Angiostrongylus cantonensis.
Authors: Tsai HC, Chung LY, Chen ER, Sy CL
Am. J. Trop. Med. Hyg., 2008-01-01;78(1):20-7.
Species: Human
Sample Types: CSF
-
Interleukin-1beta induced activation of nuclear factor-kappab can be inhibited by novel pharmacological agents in osteoarthritis.
Authors: Lauder SN, Carty SM, Carpenter CE
Rheumatology (Oxford), 2007-01-11;46(5):752-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased production of matrix metalloproteinases in Helicobacter pylori-associated human gastritis.
Authors: Bergin PJ, Anders E, Sicheng W, Erik J, Jennie A, Hans L, Pierre M, Qiang PH, Marianne QJ
Helicobacter, 2004-06-01;9(0):201.
Species: Human
Sample Types: Tissue Homogenates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human TIMP-1 DuoSet ELISA
Average Rating: 4.8 (Based on 6 Reviews)
Have you used Human TIMP-1 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
EDTA-plasma, neat. Good protocol, easy to follow and results are consistent
we used this kit to quantify human TIMP1 in serum and plasma samples. Works very well and easy to use protocol.
We ran human EDTA-plasma samples at a 1:300 dilution with high precision for this analysis.