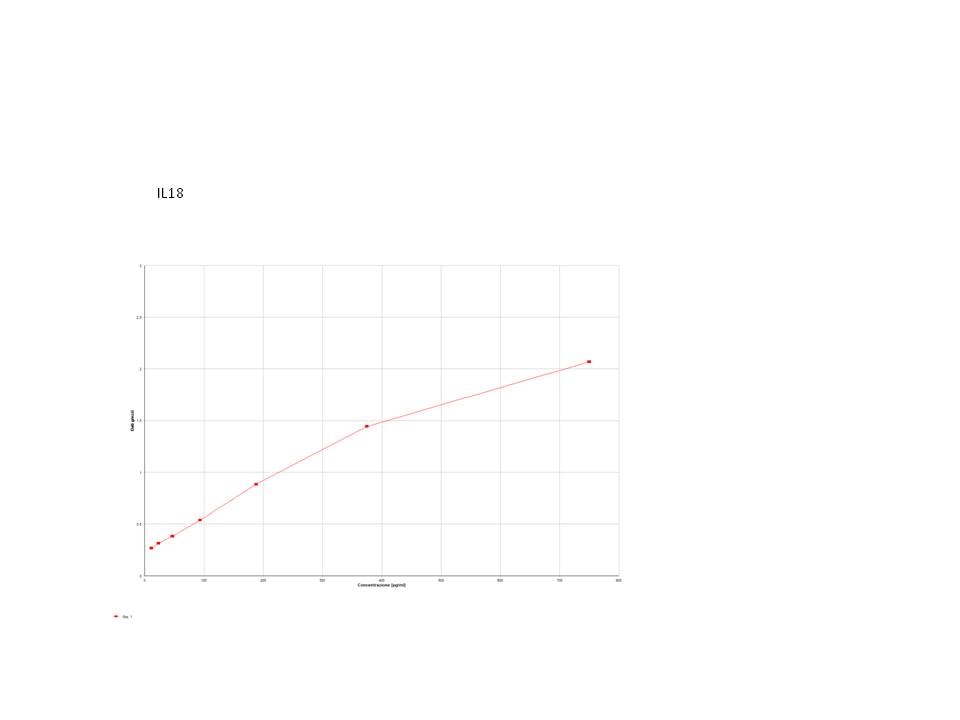
Human Total IL-18 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human Total Interleukin 18 (Total IL-18). The suggested diluent is suitable for the analysis of most cell culture supernate, serum, and plasma samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-18/IL-1F4
Interleukin 18 (IL-18), also known as interferon-gamma-inducing factor (IGIF) and IL-1γ, is a cytokine which shares biologic activities with IL-12 and structural similarities with the IL-1 family of proteins. IL-18 was originally cloned from liver cells and has since been shown to be expressed by monocyte/macrophages, osteoblasts and keratinocytes. Caspase-1 (IL-1 beta-converting enzyme) has been implicated in the physiological processing of pro-IL-18 to IL-18. Similarly to IL-12, human IL-18 has been shown to enhance NK cell activity in PBMC cultures. Human IL-18 has also been found to induce the production IFN-γ and GM-CSF while inhibiting the production of IL-10 by PBMC. On enriched human T cells, human IL-18 can enhance Th1 cytokine production and stimulate cell proliferation via an IL-2-dependent pathway. In the mouse system, IL-18 has been shown to be a costimulatory factor for the activation of Th1, but not Th2, cells. IL-18 was found to selectively enhance the FasL-mediated cytotoxicity of Th1, but not Th0 or Th2, cells. IL-18 has also been shown to induce activated B cells to produce IFN-γ that inhibits IgE production.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human Total IL-18 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
43
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Very Early-Onset IBD-Associated IL-18opathy Treated with an Anti-IL-18 Antibody
Authors: Guha, A;Diaz-Pino, R;Fagbemi, A;Hughes, SM;Wynn, RF;Lopez-Castejon, G;Arkwright, PD;
Journal of clinical medicine
Species: Human
Sample Types: Serum
-
The assembly of neutrophil inflammasomes during COVID-19 is mediated by type I interferons
Authors: Cabrera, LE;Jokiranta, ST;Mäki, S;Miettinen, S;Kant, R;Kareinen, L;Sironen, T;Pietilä, JP;Kantele, A;Kekäläinen, E;Lindgren, H;Mattila, P;Kipar, A;Vapalahti, O;Strandin, T;
PLoS pathogens
Species: Human
Sample Types: Cell Culture Supernates
-
Vaccinia virus F1L blocks the ribotoxic stress response to subvert ZAK?-dependent NLRP1 inflammasome activation
Authors: Szymanska, I;Bauernfried, S;Komar, T;Hornung, V;
European journal of immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Differentiation in pyroptosis induction by Burkholderia pseudomallei and Burkholderia thailandensis in primary human monocytes, a possible cause of sepsis in acute melioidosis patients
Authors: Khongpraphan, S;Ekchariyawat, P;Sanongkiet, S;Luangjindarat, C;Sirisinha, S;Ponpuak, M;Midoeng, P;Pudla, M;Utaisincharoen, P;
PLoS neglected tropical diseases
Species: Human
Sample Types: Cell Culture Supernates
-
Toxoplasma gondii infection associated with inflammasome activation and neuronal injury
Authors: Andreou, D;Steen, NE;Mørch-Johnsen, L;Jørgensen, KN;Wortinger, LA;Barth, C;Szabo, A;O'Connell, KS;Lekva, T;Hjell, G;Johansen, IT;Ormerod, MBEG;Haukvik, UK;Aukrust, P;Djurovic, S;Yolken, RH;Andreassen, OA;Ueland, T;Agartz, I;
Scientific reports
Species: Human
Sample Types: Plasma
-
Mucosal-Associated Invariant T Cells are not susceptible in vitro to SARS-CoV-2 infection but accumulate into the lungs of COVID-19 patients
Authors: Huang, X;Kantonen, J;Nowlan, K;Nguyen, NA;Jokiranta, ST;Kuivanen, S;Heikkilä, N;Mahzabin, S;Kantele, A;Vapalahti, O;Myllykangas, L;Heinonen, S;Mäyränpää, MI;Strandin, T;Kekäläinen, E;
Virus research
Species: Human
Sample Types: Plasma
-
Inactivation of the NLRP3 inflammasome mediates exosome-based prevention of atrial fibrillation
Authors: Parent, S;Vaka, R;St Amant, J;Kahn, S;Van Remortel, S;Bi, C;Courtman, D;Stewart, DJ;Davis, DR;
Theranostics
Species: Rat
Sample Types: Cell Culture Supernates, Cell Lysates
-
Synovial Fluid from Patients with Osteoarthritis Shows Different Inflammatory Features Depending on the Presence of Calcium Pyrophosphate Crystals
Authors: Oliviero, F;Baggio, C;Favero, M;Damasco, AC;Boscaro, C;Tietto, D;Albiero, M;Doria, A;Ramonda, R;
International journal of molecular sciences
Species: Human
Sample Types: Synovial Fluid
-
Association between cognitive function and IL-18 levels in schizophrenia: Dependent on IL18 - 607 A/C polymorphism
Authors: Guan, X;Leng, W;Hu, Q;Xiu, M;Zhang, X;
Psychoneuroendocrinology
Species: Human
Sample Types: Serum
-
No impact of a high-fat meal coupled with intermittent hypoxemia on acute kidney injury biomarkers in adults with and without obstructive sleep apnea
Authors: Goulet, N;Tetzlaff, EJ;Morin, R;Mauger, JF;Amaratunga, R;Kenny, GP;Imbeault, P;
Physiological reports
Species: Human
Sample Types: Plasma
-
Serum TGF-?1 and CD14 Predicts Response to Anti-TNF-? Therapy in IBD
Authors: Coufal, S;Kverka, M;Kreisinger, J;Thon, T;Rob, F;Kolar, M;Reiss, Z;Schierova, D;Kostovcikova, K;Roubalova, R;Bajer, L;Jackova, Z;Mihula, M;Drastich, P;Tresnak Hercogova, J;Novakova, M;Vasatko, M;Lukas, M;Tlaskalova-Hogenova, H;Jiraskova Zakostelska, Z;
Journal of immunology research
Species: Human
Sample Types: Serum
-
Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease
Authors: Fiorillo, A;Gallego, JJ;Casanova-Ferrer, F;Giménez-Garzó, C;Urios, A;Ballester, MP;Durbán, L;Rios, MP;Megías, J;San Miguel, T;Kosenko, E;Escudero-García, D;Benlloch, S;Felipo, V;Montoliu, C;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
NK cell-derived extracellular granzyme B drives epithelial ulceration during HSV-2 genital infection
Authors: YS Lim, AG Lee, X Jiang, JM Scott, A Cofie, S Kumar, D Kennedy, DJ Granville, H Shin
Cell Reports, 2023-04-17;42(4):112410.
Species: Human
Sample Types: Vaginal Lavage Fluid
-
CARD-only proteins regulate in�vivo inflammasome responses and ameliorate gout
Authors: S Devi, M Indramohan, E Jäger, J Carriere, LH Chu, L de Almeida, DR Greaves, C Stehlik, A Dorfleutne
Cell Reports, 2023-03-16;42(3):112265.
Species: Human
Sample Types: Cell Culture Supernates
-
Imbalanced IL-1B and IL-18 Expression in S�zary Syndrome
Authors: KCG Manfrere, MP Torrealba, FM Ferreira, ESA de Sousa, D Miyashiro, FME Teixeira, RWA Custódio, HI Nakaya, YAL Ramos, MN Sotto, A Woetmann, N Ødum, AJDS Duarte, JA Sanches, MN Sato
International Journal of Molecular Sciences, 2023-02-28;24(5):.
Species: Human
Sample Types: Serum
-
CXCL12 and CXCR4 as Novel Biomarkers in Uric Acid-Induced Inflammation and Patients with Gouty Arthritis
Authors: SK Kim, JY Choe, KY Park
Biomedicines, 2023-02-21;11(3):.
Species: Human
Sample Types: Serum
-
Hepatocyte phosphatase DUSP22 mitigates NASH-HCC progression by targeting FAK
Authors: C Ge, J Tan, X Dai, Q Kuang, S Zhong, L Lai, C Yi, Y Sun, J Luo, C Zhang, L Zhu, B Wang, M Xu
Oncogene, 2022-10-08;13(1):5945.
Species: Human
Sample Types: Serum
-
Interleukin-18 signaling system links to agitation in severe mental disorders
Authors: G Hjell, A Szabo, L Mørch-John, R Holst, N Tesli, C Bell, T Fischer-Vi, MCF Werner, SH Lunding, MBEG Ormerod, IT Johansen, I Dieset, S Djurovic, I Melle, T Ueland, OA Andreassen, NE Steen, UK Haukvik
Psychoneuroendocrinology, 2022-03-12;140(0):105721.
Species: Human
Sample Types: Serum
-
The role of caspase-1, caspase-4 and NLRP3 in regulating the host cell response evoked by uropathogenic Escherichia coli
Authors: A Lindblad, C Johansson, K Persson, I Demirel
Scientific Reports, 2022-02-07;12(1):2005.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical and biological markers for predicting ARDS and outcome in septic patients
Authors: J Villar, R Herrán-Mon, E González-H, M Prieto-Gon, A Ambrós, A Rodríguez-, A Muriel-Bom, R Solano, C Cuenca-Rub, A Vidal, C Flores, JM González-M, MI García-Lao, Genetics o
Scientific Reports, 2021-11-22;11(1):22702.
Species: Human
Sample Types: Serum
-
Topical inflammasome inhibition with disulfiram prevents irritant contact dermatitis
Authors: H Bonnekoh, C Vera, A Abad-Perez, S Radetzki, M Neuenschwa, E Specker, NA Mahnke, S Frischbutt, E Latz, M Nazaré, JV Kries, M Maurer, J Scheffel, K Krause
Clinical and translational allergy, 2021-07-22;11(5):e12045.
Species: Human
Sample Types: Tissue Homogenates
-
DHX15 is required to control RNA virus-induced intestinal inflammation
Authors: J Xing, X Zhou, M Fang, E Zhang, LJ Minze, Z Zhang
Cell Reports, 2021-06-22;35(12):109205.
Species: Human
Sample Types: Cell Culture Supernates
-
Lipopolysaccharide stimulation test on cultured PBMCs assists the discrimination of cryopyrin-associated periodic syndrome from systemic juvenile idiopathic arthritis
Authors: CY Wu, WL Fan, YM Chiu, HY Yang, WI Lee, JL Huang
Scientific Reports, 2021-06-07;11(1):11903.
Species: Human
Sample Types: Plasma
-
Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by SARS-CoV-2 Spike Protein
Authors: E Jover, L Matilla, M Garaikoetx, A Fernández-, P Muntendam, F Jaisser, P Rossignol, N López-Andr
Biomedicines, 2021-06-03;9(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of Inflammatory Immune Dysfunction in Psoriasis Patients at Risk for COVID-19
Authors: TM Yendo, MN Sato, ACCC Branco, AJ Pietrobon, FME Teixeira, YÁL Ramos, RW Alberca, CG Valêncio, VN Arruda, R Romiti, M Arnone, ALDS Hirayama, AJDS Duarte, V Aoki, RL Orfali
Vaccines, 2021-05-10;9(5):.
Species: Human
Sample Types: Plasma
-
Differential recognition of HIV-stimulated IL-1&beta and IL-18 secretion through NLR and NAIP signalling in monocyte-derived macrophages
Authors: K Triantafil, CJK Ward, M Czubala, RG Ferris, E Koppe, C Haffner, V Piguet, VK Patel, H Amrine-Mad, L Modis, SL Masters, M Triantafil
PloS Pathogens, 2021-04-16;17(4):e1009417.
Species: Human
Sample Types: Cell Culture Supernates
-
The Role of Endothelins, IL-18, and NGAL in Kidney Hypothermic Machine Perfusion
Authors: K Tejchman, A Nowacki, K Kotfis, E Skwirczyns, M Kotowski, L Zair, M Ostrowski, J Sienko
Biomedicines, 2021-04-13;9(4):.
Species: Human
Sample Types: Plasma
-
Propionibacterium acnes Accelerates Intervertebral Disc Degeneration by Inducing Pyroptosis of Nucleus Pulposus Cells via the ROS-NLRP3 Pathway
Authors: G Tang, X Han, Z Lin, H Qian, B Chen, C Zhou, Y Chen, W Jiang
Oxidative Medicine and Cellular Longevity, 2021-02-01;2021(0):4657014.
Species: Human
Sample Types: Cell Culture Supernates
-
Early bilirubinemia after allogeneic stem cell transplantation-an endothelial complication
Authors: H Dai, O Penack, A Radujkovic, D Schult, J Majer-Laut, IW Blau, L Bullinger, S Jiang, C Müller-Tid, P Dreger, T Luft
Bone marrow transplantation, 2021-01-30;0(0):.
Species: Human
Sample Types: Serum
-
Clinical Response to the CD95-Ligand Inhibitor Asunercept Is Defined by a Pro-Inflammatory Serum Cytokine Profile
Authors: A Radujkovic, T Boch, F Nolte, D Nowak, C Kunz, A Gieffers, C Müller-Tid, P Dreger, WK Hofmann, T Luft
Cancers, 2020-12-08;12(12):.
Species: Human
Sample Types: Serum
-
Interleukin-18 and Hematopoietic Recovery after Allogeneic Stem Cell Transplantation
Authors: A Radujkovic, L Kordelas, R Bogdanov, C Müller-Tid, DW Beelen, P Dreger, T Luft
Cancers (Basel), 2020-09-28;12(10):.
Species: Human
Sample Types: Serum
-
The Delivery of &alpha1-Antitrypsin Therapy Through Transepidermal Route: Worthwhile to Explore
Authors: S Tumpara, B Martinez-D, G Gomez-Mari, B Liu, DS DeLuca, E Korenbaum, D Jonigk, F Jugert, FM Wurm, MJ Wurm, T Welte, S Janciauski
Front Pharmacol, 2020-07-03;11(0):983.
Species: Human
Sample Types: Cell Culture Supernates
-
Design and Characterization of an "All-in-One" Lentiviral Vector System Combining Constitutive Anti-GD2 CAR Expression and Inducible Cytokines
Authors: K Zimmermann, J Kuehle, AC Dragon, M Galla, C Kloth, LS Rudek, IE Sandalciog, B Neyazi, T Moritz, J Meyer, C Rossig, B Altvater, B Eiz-Vesper, MA Morgan, H Abken, A Schambach
Cancers (Basel), 2020-02-06;12(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Quartz Dust Exposure Affects NLRP3 Inflammasome Activation and Plasma Levels of IL-18 and IL-1Ra in Iron Foundry Workers
Authors: A Hedbrant, L Andersson, IL Bryngelsso, D Eklund, H Westberg, E Särndahl, A Persson
Mediators Inflamm., 2020-01-07;2020(0):8490908.
Species: Human
Sample Types: Plasma
-
Increased interleukin 18 activity in adolescents with early-onset psychosis is associated with cortisol and depressive symptoms
Authors: K Wedervang-, S Friis, V Lonning, RE Smelror, C Johannesse, EJ Reponen, SH Lyngstad, T Lekva, P Aukrust, T Ueland, OA Andreassen, I Agartz, AM Myhre
Psychoneuroendocrinology, 2019-11-14;0(0):104513.
Species: Human
Sample Types: Serum
-
EASIX for prediction of survival in lower-risk myelodysplastic syndromes
Authors: A Merz, U Germing, G Kobbe, J Kaivers, A Jauch, A Radujkovic, M Hummel, A Benner, M Merz, P Dreger, T Luft
Blood Cancer J, 2019-11-11;9(11):85.
Species: Human
Sample Types: Plasma
-
Lysosomotropic beta blockers induce oxidative stress and IL23A production in Langerhans cells
Authors: G Müller, C Lübow, G Weindl
Autophagy, 2019-11-07;0(0):1-16.
Species: Human
Sample Types: Cell Culture Supernatants
-
Human metapneumovirus activates NOD-like receptor protein 3 inflammasome via its small hydrophobic protein which plays a detrimental role during infection in mice
Authors: VB Lê, J Dubois, C Couture, MH Cavanagh, O Uyar, A Pizzorno, M Rosa-Calat, MÈ Hamelin, G Boivin
PLoS Pathog., 2019-04-09;15(4):e1007689.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of constitutional TET2 haploinsufficiency on molecular and clinical phenotype in humans
Authors: E Kaasinen, O Kuismin, K Rajamäki, H Ristolaine, M Aavikko, J Kondelin, S Saarinen, DG Berta, R Katainen, EAM Hirvonen, A Karhu, A Taira, T Tanskanen, A Alkodsi, M Taipale, E Morgunova, K Franssila, R Lehtonen, M Mäkinen, K Aittomäki, A Palotie, MI Kurki, O Pietiläine, M Hilpert, E Saarentaus, J Niinimäki, J Junttila, K Kaikkonen, P Vahteristo, RC Skoda, MRJ Seppänen, KK Eklund, J Taipale, O Kilpivaara, LA Aaltonen
Nat Commun, 2019-03-19;10(1):1252.
Species: Human
Sample Types: Cell Culture Supernates
-
Asymmetric dimethylarginine serum levels are associated with early mortality after allogeneic stem cell transplantation
Authors: A Radujkovic, H Dai, L Kordelas, D Beelen, SP Rachakonda, C Müller-Tid, R Kumar, P Dreger, T Luft
Haematologica, 2018-12-04;0(0):.
Species: Human
Sample Types: Serum
-
TET-2 up-regulation is associated with the anti-inflammatory action of Vicenin-2
Authors: N Hassan, A Ali, C Withycombe, M Ahluwalia, RH Al-Nasseri, A Tonks, K Morris
Cytokine, 2018-03-19;108(0):37-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Altered distribution of peripheral blood dendritic cell subsets in patients with pulmonary paracoccidioidomycosis
Authors: J Venturini, RS Cavalcante, DV Moris, M de Assis G, AD Levorato, KH Dos Reis, MSP de Arruda, RP Mendes
Acta Trop., 2017-06-09;0(0):.
Species: Human
Sample Types: Serum
-
TB lymphadenitis is associated with enhanced baseline and antigen - specific induction of Type 1 and Type 17 cytokines and reduced IL-1? and IL-18 at the site of infection
Authors: GR Kathamuthu, K Moideen, D Baskaran, VV Banurekha, D Nair, G Sekar, R Sridhar, B Vidyajayan, G Gajendrara, DK Parandhama, A Srinivasan, S Babu
Clin. Vaccine Immunol, 2017-05-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human Total IL-18 DuoSet ELISA
Average Rating: 5 (Based on 10 Reviews)
Have you used Human Total IL-18 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
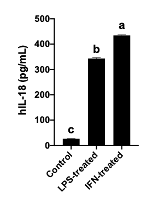
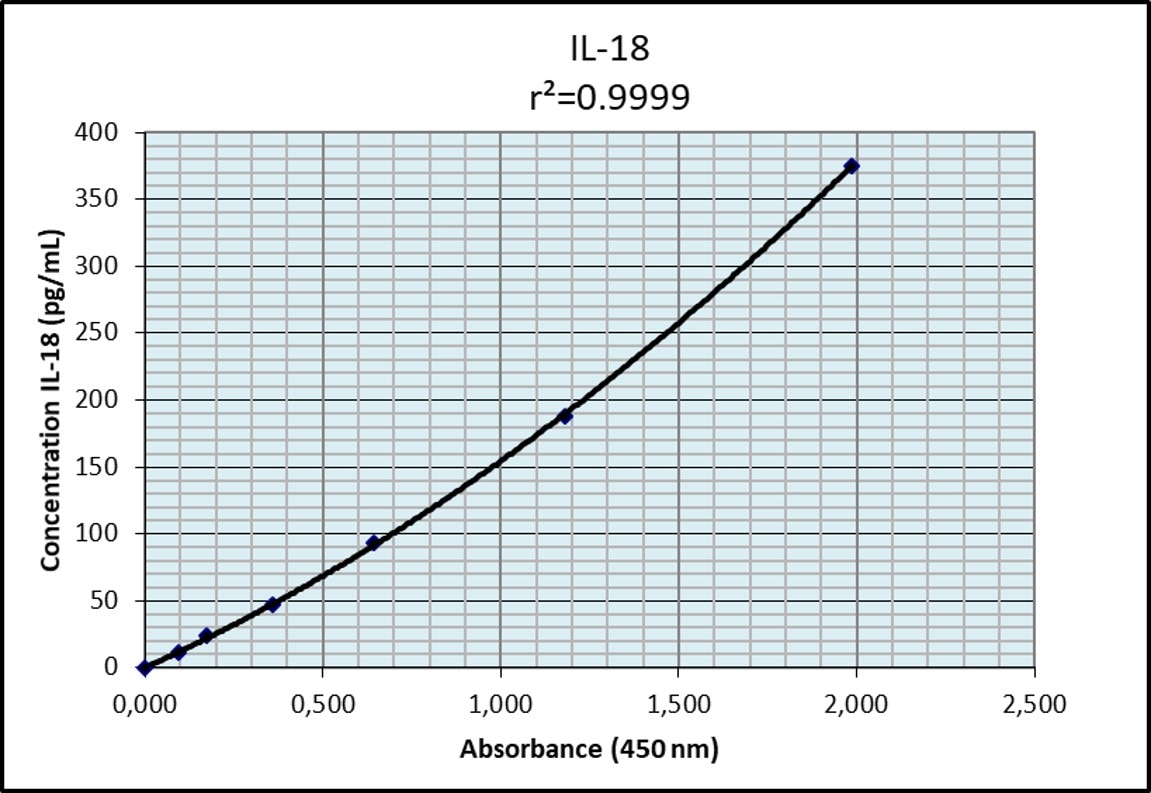
The kit was essential to quantify this cytokine in Caco-2 cells, previously grown for 21 days and then treated with pro-inflammatory factors (LPS and IFN-gamma). Results obtained agreed with other reports in the literature and were highly replicable.
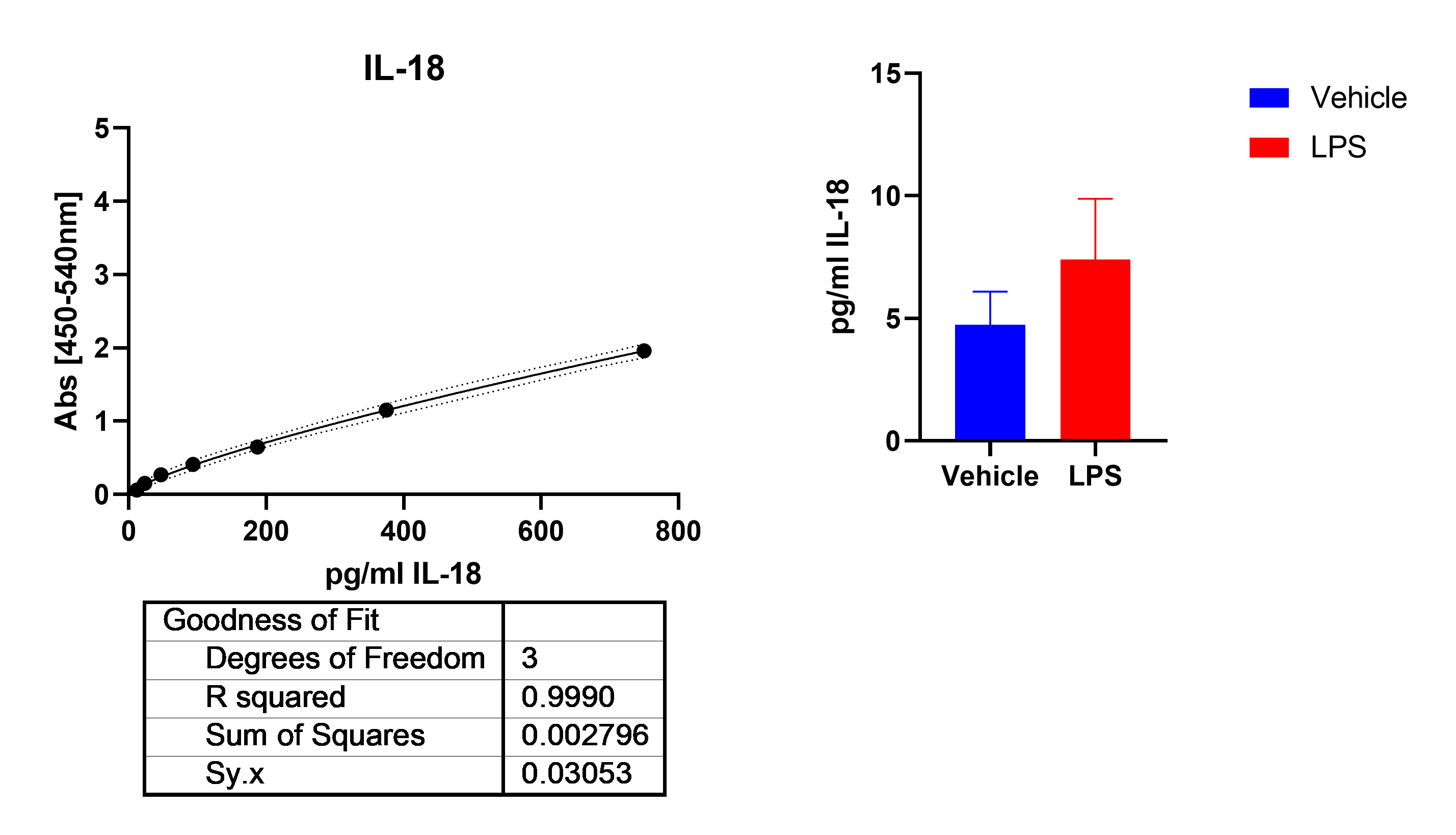
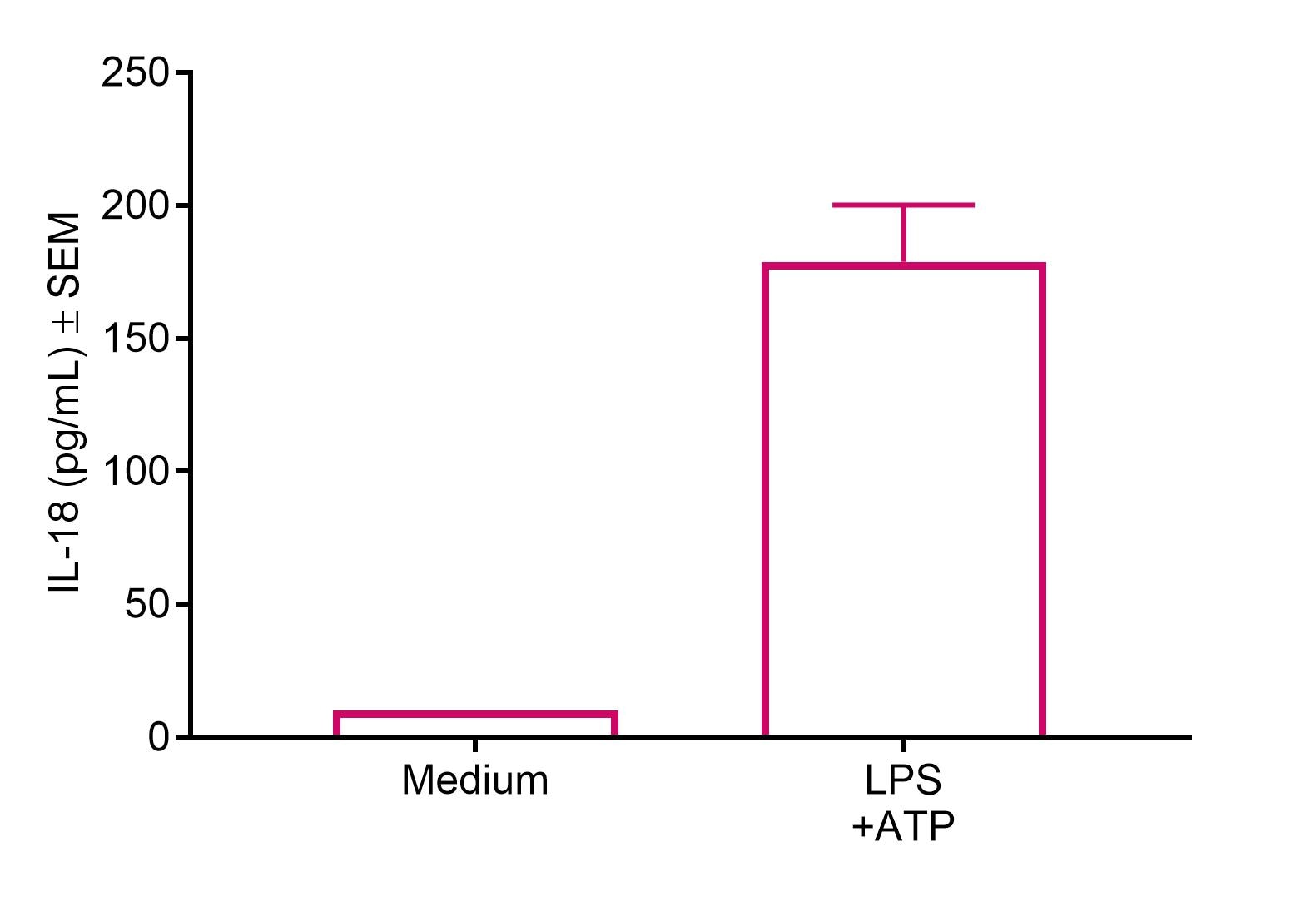
HLF cells treated with LPS+ATP
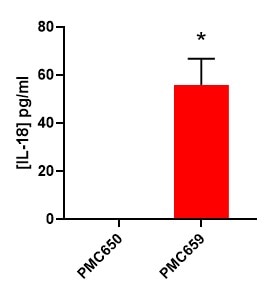
Detection of IL-18 produced by human CAR-T cells engineered to express IL-18 (PMC659), compared to normal CAR-T cells (PMC650).
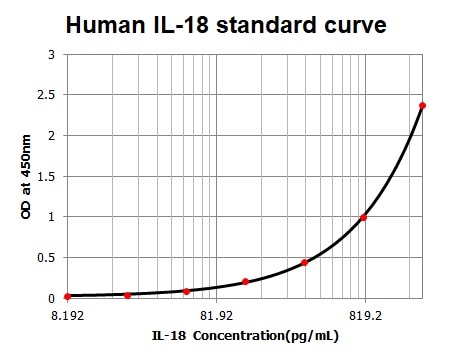
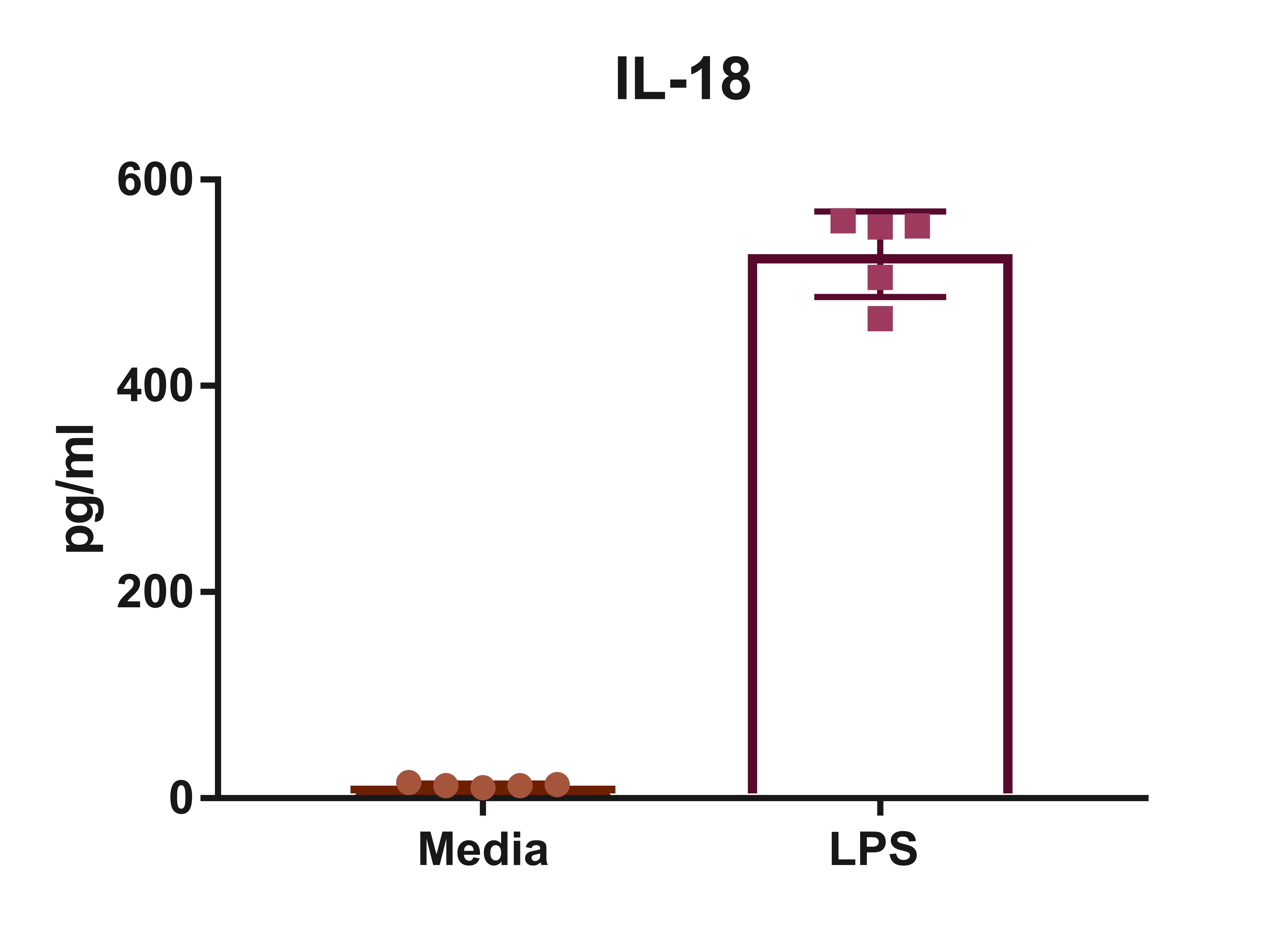
The PMA-differentiated THP-1 cells were treated with or without LPS/ATP for 4 h. The production of IL-18 was tested from culture supernatants of treated THP-1 cells.
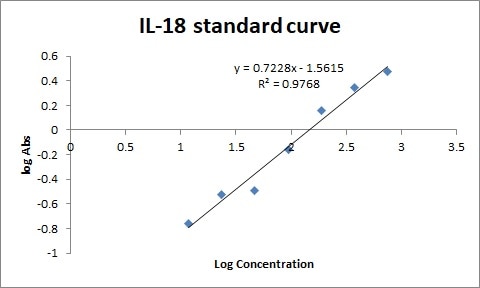
We used human serum at a dilution of 1:10. The result was very good.