Human VEGF DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human VEGF. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Customers also Viewed
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
 View Larger
View Larger
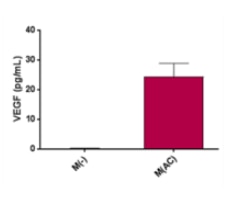
Human VEGF DuoSet ELISA Effects of cell stress on VEGF-A and HIF1 alpha expression in Müller cells and Y79 photoreceptors. (A) VEGF-A was measured by ELISA using conditioned media collected from MIO-M1 and Y79 cells, n = 4–5/group in Müller cells and n = 8/group in Y79 photoreceptors, ** p < 0.01; (B) Western blots for HIF1 alpha using cellular proteins, n = 4/group, ** p < 0.01. For MIO-M1 Müller cells: Grey bars: 5 mM glucose. Black bars: 25 mM glucose. For Y79 photoreceptors: Grey bars: 11 mM glucose. Black bars: 25 mM glucose. NS, not significant; LG, low glucose; HG, high glucose. Image collected and cropped by CiteAb from the following open publication (https://www.mdpi.com/1422-0067/18/3/533), licensed under a CC-BY license. Not internally tested by R&D Systems.
Product Datasheets
Preparation and Storage
Background: VEGF
Vascular endothelial growth factor (VEGF or VEGF-A), also known as vascular permeability factor (VPF), is a potent mediator of both angiogenesis and vasculogenesis in the fetus and adult (1-3). It is a member of the PDGF family that is characterized by the presence of eight conserved cysteine residues in a cystine knot structure and the formation of antiparallel disulfide-linked dimers (4). Humans express alternately spliced isoforms of 121, 145, 165, 183, 189, and 206 amino acids (aa) in length (4). VEGF165 appears to be the most abundant and potent isoform, followed by VEGF121 and VEGF189 (3, 4). Isoforms other than VEGF121 contain basic heparin-binding regions and are not freely diffusible (4). Human VEGF165 shares 88% aa sequence identity with corresponding regions of mouse and rat VEGF. VEGF is expressed in multiple cells and tissues including skeletal and cardiac muscle (5, 6), hepatocytes (7), osteoblasts (8), neutrophils (9), macrophages (10), keratinocytes (11), brown adipose tissue (12), CD34+ stem cells (13), endothelial cells (14), fibroblasts, and vascular smooth muscle cells (15). VEGF expression is induced by hypoxia and cytokines such as IL-1, IL-6, IL-8, oncostatin M, and TNF-alpha (3, 4, 9, 16). VEGF isoforms are differentially expressed during development and in the adult (3).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human VEGF DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
198
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
PCRX-201, a novel IL-1Ra gene therapy treatment approach for low back pain resulting from intervertebral disc degeneration
Authors: Snuggs, JW;Senter, RK;Whitt, JP;Jackson, JD;Le Maitre, CL;
Gene therapy
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of RNA editing and gene therapy with a compact CRISPR-Cas13 in the retina
Authors: Kumar, S;Hsiao, YW;Wong, VHY;Aubin, D;Wang, JH;Lisowski, L;Rakoczy, EP;Li, F;Alarcon-Martinez, L;Gonzalez-Cordero, A;Bui, BV;Liu, GS;
Proceedings of the National Academy of Sciences of the United States of America
Species: Human
Sample Types: Cell Culture Supernates
-
Clustered ARPE-19 cells distinct in mitochondrial membrane potential may play a pivotal role in cell differentiation
Authors: Miyatani, T;Tanaka, H;Numa, K;Uehara, A;Otsuki, Y;Hamuro, J;Kinoshita, S;Sotozono, C;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Neuroprotective effects of intranasal extracellular vesicles from human platelet concentrates supernatants in traumatic brain injury and Parkinson's disease models
Authors: Delila, L;Nebie, O;Le, NTN;Timmerman, K;Lee, DY;Wu, YW;Chou, ML;Buée, L;Chou, SY;Blum, D;Devos, D;Burnouf, T;
Journal of biomedical science
Species: Human
Sample Types: Extracellular Vesicles
-
Loss of Tuberous Sclerosis Complex 2 confers inflammation via dysregulation of Nuclear factor kappa-light-chain-enhancer of activated B cells
Authors: McPhail, DK;Alzahrani, MAM;Martin, KR;Calver, BL;Harwood, AJ;MacKeigan, JP;Davies, DM;Tee, AR;
Research square
Species: Human
Sample Types: Cell Culture Supernates
-
Glycogen myophosphorylase loss causes increased dependence on glucose in iPSC-derived retinal pigment epithelium
Authors: Basu, B;Karwatka, M;China, B;McKibbin, M;Khan, K;Inglehearn, CF;Ladbury, JE;Johnson, CA;
The Journal of biological chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Tofacitinib Regulates Endostatin via Effects on CD147 and Cathepsin S
Authors: Zisman, D;Sabtan, H;Rahat, MM;Simanovich, E;Haddad, A;Gazitt, T;Feld, J;Slobodin, G;Kibari, A;Elias, M;Rahat, MA;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
The Reduction of PSMB4 in T24 and J82 Bladder Cancer Cells Inhibits the Angiogenesis and Migration of Endothelial Cells
Authors: Lin, YH;Chen, TM;Tsai, YL;Tsai, WC;Wang, HH;Chen, Y;Wu, ST;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Bone Regenerative Effect of Injectable Hypoxia Preconditioned Serum-Fibrin (HPS-F) in an Ex Vivo Bone Defect Model
Authors: Jiang, J;Röper, L;Fuchs, F;Hanschen, M;Failer, S;Alageel, S;Cong, X;Dornseifer, U;Schilling, AF;Machens, HG;Moog, P;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
Investigating the paracrine and juxtacrine abilities of adipose-derived stromal cells in angiogenesis triple cell co-cultures
Authors: Harary Søndergaard, R;Drozd Højgaard, L;Haack-Sørensen, M;Hoeeg, C;Mønsted Johansen, E;Follin, B;Kastrup, J;Ekblond, A;Juhl, M;
Stem cell research
Species: Human
Sample Types: Cell Culture Supernates
-
Glucose Promotes EMMPRIN/CD147 and the Secretion of Pro-Angiogenic Factors in a Co-Culture System of Endothelial Cells and Monocytes
Authors: Ghandour, F;Kassem, S;Simanovich, E;Rahat, M;
Biomedicines
Species: Human
Sample Types: Cell Culture Supernates
-
Assessment of the Integrity and Function of Human Term Placental Explants in Short-Term Culture
Authors: López-Guzmán, C;García, AM;Marín, P;Vásquez, AM;
Methods and protocols
Species: Human
Sample Types: Cell Culture Supernates
-
High FSH levels impair VEGF secretion of human, frozen-thawed ovarian cortical tissue in vitro
Authors: Einenkel, R;Schallmoser, A;Sänger, N;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Exploring the potential use of platelet rich plasma (PRP) from adult and umbilical cord blood in murine follicle culture
Authors: Subiran Adrados, C;Cadenas, J;Polat, SL;Tjäder, AS;Blanche, P;Kristensen, SG;
Reproductive biology
Species: Human
Sample Types: Platelet Lysates
-
Ventilatory support and inflammatory peptides in hospitalised patients with COVID-19: A prospective cohort trial
Authors: Gysan, MR;Milacek, C;Bal, C;Zech, A;Brugger, J;Milos, RI;Antoniewicz, L;Idzko, M;Gompelmann, D;
PloS one
Species: Human
Sample Types: Serum
-
Unrevealed roles of extracellular enolase?1 (ENO1) in promoting glycolysis and pro?cancer activities in multiple myeloma via hypoxia?inducible factor 1?
Authors: Chung, IC;Huang, WC;Huang, YT;Chen, ML;Tsai, AW;Wu, PY;Yuan, TT;
Oncology reports
Species: Human, Mouse
Sample Types: Cell Culture Supernates
-
Surgical Site-Released Tissue Is Potent to Generate Bone onto TCP and PCL-TCP Scaffolds In Vitro
Authors: Rehage, E;Sowislok, A;Busch, A;Papaeleftheriou, E;Jansen, M;Jäger, M;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
CD40 Ligand-CD40 Interaction Is an Intermediary between Inflammation and Angiogenesis in Proliferative Diabetic Retinopathy
Authors: Abu El-Asrar, AM;Nawaz, MI;Ahmad, A;Dillemans, L;Siddiquei, M;Allegaert, E;Gikandi, PW;De Hertogh, G;Opdenakker, G;Struyf, S;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates, Vitreous Humor
-
Adipose stromal cells bioproducts as cell-free therapies: manufacturing and therapeutic dose determine in vitro functionality
Authors: Skovronova, R;Scaccia, E;Calcat-I-Cervera, S;Bussolati, B;O'Brien, T;Bieback, K;
Journal of translational medicine
Species: Human
Sample Types: Cell Lysates
-
Transplantation of adipose tissue-derived microvascular fragments promotes therapy of critical limb ischemia
Authors: Park, GT;Lim, JK;Choi, EB;Lim, MJ;Yun, BY;Kim, DK;Yoon, JW;Hong, YG;Chang, JH;Bae, SH;Ahn, JY;Kim, JH;
Biomaterials research
Species: Human
Sample Types: Cell Culture Supernates
-
Harnessing EGLN1 Gene Editing to Amplify HIF-1 alpha and Enhance Human Angiogenic Response
Authors: Shams, S;Stilhano, RS;Silva, EA;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Contribution of Mesenchymal Stem Cells from Obese Adipose Tissue to PD-L1 Over-Expression and Breast Cancer Progression through Pathogenic Th17 Cell Activation
Authors: Blangero, F;Robert, M;Andraud, T;Dumontet, C;Vidal, H;Eljaafari, A;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
Differential Impact of Intermittent vs. Sustained Hypoxia on HIF-1, VEGF and Proliferation of HepG2 Cells
Authors: M Minoves, F Hazane-Puc, G Moriondo, A Boutin-Par, E Lemarié, JL Pépin, D Godin-Ribu, A Briançon-M
International Journal of Molecular Sciences, 2023-04-07;24(8):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA -
Nailfold Videocapillaroscopy for Non-Invasive Assessment of Microcirculation and Prognostic Correlation with Endothelial Dysfunction, Cardiovascular Risk Factors, and Non-HLA Antibodies in Heart Transplant Recipients: A Pilot Study
Authors: D Sikorska, D Kami?ska, R Catar, D Wu, H Zhao, P Wang, J Kamhieh-Mi, M Banasik, M Kusztal, M Cielecka, M Zakliczy?s, R Rutkowski, K Korybalska, H Heidecke, G Moll, W Samborski
Journal of Clinical Medicine, 2023-03-16;12(6):.
Species: Human
Sample Types: Serum
-
The Antimicrobial Peptide Cathelicidin Exerts Immunomodulatory Effects via Scavenger Receptors
Authors: R Amagai, T Takahashi, H Terui, T Fujimura, K Yamasaki, S Aiba, Y Asano
International Journal of Molecular Sciences, 2023-01-03;24(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of Exercise Training on Neurotrophic Factors and Blood-Brain Barrier Permeability in Young-Old and Old-Old Women
Authors: SY Cho, HT Roh
International Journal of Environmental Research and Public Health, 2022-12-16;19(24):.
Species: Human
Sample Types: Serum
-
Macrophage NFATC2 mediates angiogenic signaling during mycobacterial infection
Authors: WJ Brewer, AM Xet-Mull, A Yu, MI Sweeney, EM Walton, DM Tobin
Cell Reports, 2022-12-13;41(11):111817.
Species: Human
Sample Types: Cell Culture Supernates
-
Prostacyclin Released by Cancer-Associated Fibroblasts Promotes Immunosuppressive and Pro-Metastatic Macrophage Polarization in the Ovarian Cancer Microenvironment
Authors: L Sommerfeld, I Knuth, F Finkernage, J Pesek, WA Nockher, JM Jansen, U Wagner, A Nist, T Stiewe, S Müller-Brü, R Müller, S Reinartz
Cancers, 2022-12-13;14(24):.
Species: Human
Sample Types:
-
Paracrine and Autocrine Effects of VEGF Are Enhanced in Human eMSC Spheroids
Authors: I Kozhukharo, N Minkevich, L Alekseenko, A Domnina, O Lyublinska
International Journal of Molecular Sciences, 2022-11-18;23(22):.
Species: Human
Sample Types: Cell Culture Supernates
-
KDM6A Loss Recruits Tumor-Associated Neutrophils and Promotes Neutrophil Extracellular Trap Formation in Pancreatic Cancer
Authors: J Yang, L Jin, HS Kim, F Tian, Z Yi, K Bedi, M Ljungman, M Pasca di M, H Crawford, J Shi
Cancer Research, 2022-11-15;0(0):OF1-OF14.
Species: Human
Sample Types: Cell Culture Supernates
-
Angiogenic Potential of Human Adipose-Derived Mesenchymal Stromal Cells in Nanofibrillated Cellulose Hydrogel
Authors: E Koivunotko, J Snirvi, A Merivaara, R Harjumäki, S Rautiainen, M Kelloniemi, K Kuismanen, S Miettinen, M Yliperttul, R Koivuniemi
Biomedicines, 2022-10-15;10(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Growth Factor Release within Liquid and Solid PRF
Authors: K Zwittnig, B Kirnbauer, N Jakse, P Schlenke, I Mischak, S Ghanaati, S Al-Maawi, D Végh, M Payer, TA Zrnc
Journal of Clinical Medicine, 2022-08-29;11(17):.
Species: Human
Sample Types: Serum
-
Pro-cancerogenic effects of spontaneous and drug-induced senescence of ovarian cancer cells in vitro and in vivo: a comparative analysis
Authors: S Rutecki, P Szulc, M Paku?a, P Uruski, A Radziemski, E Naumowicz, R Moszy?ski, A Tykarski, J Miku?a-Pie, K Ksi??ek
Journal of ovarian research, 2022-07-26;15(1):87.
Species: Human
Sample Types: Cell Culture Supernates
-
Kallistatin prevents ovarian hyperstimulation syndrome by regulating vascular leakage
Authors: J Huang, Y Mao, Q Li, H Hong, N Tang, X Kang, Y Huang, J Liu, Q Gong, Y Yao, L Li
Journal of Cellular and Molecular Medicine, 2022-07-21;26(16):4613-4623.
Species: Human
Sample Types: Cell Culture Supernates
-
VEGF-Mediated Augmentation of Autophagic and Lysosomal Activity in Endothelial Cells Defends against Intracellular Streptococcus pyogenes
Authors: SL Lu, H Omori, Y Zhou, YS Lin, CC Liu, JJ Wu, T Noda
MBio, 2022-07-05;0(0):e0123322.
Species: Human
Sample Types: Serum
-
VEGF-C serum level is associated with response to bevacizumab maintenance therapy in primary ovarian cancer patients
Authors: Y Ding, L Oliveira-F, E Vettorazzi, K Legler, K Milde-Lang, L Woelber, A Jaeger, K Prieske, V Mueller, B Schmalfeld, S Kuerti
PLoS ONE, 2022-06-10;17(6):e0269680.
Species: Human
Sample Types: Serum
-
Sirtuin 1 Induces Choroidal Neovascularization and Triggers Age-Related Macular Degeneration by Promoting LCN2 through SOX9 Deacetylation
Authors: S Zhao, Z Huang, H Jiang, J Xiu, L Zhang, Q Long, Y Yang, L Yu, L Lu, H Gu
Oncogene, 2022-06-09;2022(0):1671438.
Species: Human
Sample Types: Cell Culture Supernates
-
Nerve Growth Factor (NGF) as Partaker in the Modulation of UV-Response in Cultured Human Conjunctival Fibroblasts
Authors: G Esposito, BO Balzamino, ML Rocco, L Aloe, A Micera
International Journal of Molecular Sciences, 2022-06-06;23(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Mg-based materials diminish tumor spreading and cancer metastases
Authors: P Globig, R Madurawala, R Willumeit-, F Martini, E Mazzoni, BJC Luthringer
Bioactive materials, 2022-05-10;19(0):594-610.
Species: Human
Sample Types: Cell Culture Supernates
-
Therapeutic Effects of Platelet-Derived Extracellular Vesicles in a Bioengineered Tendon Disease Model
Authors: AL Graça, RMA Domingues, I Calejo, M Gómez-Flor, ME Gomes
International Journal of Molecular Sciences, 2022-03-09;23(6):.
Species: Human
Sample Types: Extracellular Vesicles
-
Anti-inflammatory effects of an autologous gold-based serum therapy in osteoarthritis patients
Authors: J Feldt, AJ Donaubauer, J Welss, U Schneider, US Gaipl, F Paulsen
Scientific Reports, 2022-03-03;12(1):3560.
Species: Human
Sample Types: Serum
-
Depolymerization of fucoidan with endo-fucoidanase changes bioactivity in processes relevant for bone regeneration
Authors: J Ohmes, MD Mikkelsen, TT Nguyen, VHN Tran, S Meier, MS Nielsen, M Ding, A Seekamp, AS Meyer, S Fuchs
Carbohydrate polymers, 2022-02-24;286(0):119286.
Species: Human
Sample Types: Cell Culture Supernates
-
Nucleoporin-93 reveals a common feature of aggressive breast cancers: robust nucleocytoplasmic transport of transcription factors
Authors: NB Nataraj, A Noronha, JS Lee, S Ghosh, HR Mohan Raju, A Sekar, B Zuckerman, M Lindzen, E Tarcitano, S Srivastava, M Selitrenni, I Livneh, D Drago-Garc, O Rueda, C Caldas, S Lev, T Geiger, A Ciechanove, I Ulitsky, R Seger, E Ruppin, Y Yarden
Cell Reports, 2022-02-22;38(8):110418.
Species: Human
Sample Types: Cell Culture Supernates
-
Non-HLA Antibodies in Hand Transplant Recipients Are Connected to Multiple Acute Rejection Episodes and Endothelial Activation
Authors: D Sikorska, D Kami?ska, R Catar, M Banasik, H Heidecke, K Schulze-Fo, K Korybalska, R Rutkowski, J ?uczak, J Jab?ecki, A Oko, P Daroszewsk, M Kusztal, W Samborski
Journal of Clinical Medicine, 2022-02-04;11(3):.
Species: Human
Sample Types: Serum
-
MiR-612, miR-637, and miR-874 can Regulate VEGFA Expression in Hepatocellular Carcinoma Cell Lines
Authors: MMU Castanhole, NM Tunissioll, ARCP Oliveira, MF Mattos, ALS Galbiatti-, RS Kawasaki-O, EC Pavarino, RF da Silva, EM Goloni-Ber
Genes, 2022-01-30;13(2):.
Species: Human
Sample Types: Cell Lysates
-
Circulating Interleukin-6 and CD16 positive monocytes increase following angioplasty of an arteriovenous fistula
Authors: S Hakki, EJ Robinson, MG Robson
Scientific Reports, 2022-01-26;12(1):1427.
Species: Human
Sample Types: Plasma
-
Kidney injury biomarkers and parasitic loads of Schistosoma mansoni in a highly endemic area in northeastern Brazil
Authors: RLF Galvão, GC Meneses, MCC Pinheiro, AMC Martins, EF Daher, FSM Bezerra
Acta tropica, 2022-01-14;0(0):106311.
Species: Human
Sample Types: Urine
-
Tissue Inhibitor of Metalloproteinase-3 Ameliorates Diabetes-Induced Retinal Inflammation
Authors: AM Abu El-Asr, A Ahmad, MI Nawaz, MM Siddiquei, A De Zutter, L Vanbrabant, PW Gikandi, G Opdenakker, S Struyf
Frontiers in Physiology, 2022-01-10;12(0):807747.
Species: Human
Sample Types: Serum
-
Delivery of affordable and scalable encapsulated allogenic/autologous mesenchymal stem cells in coagulated platelet poor plasma for dental pulp regeneration
Authors: I Angelopoul, C Trigo, MI Ortuzar, J Cuenca, C Brizuela, M Khoury
Scientific Reports, 2022-01-10;12(1):435.
Species: Human
Sample Types: Cell Culture Supernates
-
Mesenchymal stem cell-encapsulated cellulose nanofiber microbeads and enhanced biological activities by hyaluronic acid incorporation
Authors: M Goh, G Tae
Carbohydrate polymers, 2021-12-24;280(0):119026.
Species: Human
Sample Types: Cell Culture Supernates
-
Microfluidic Single-Cell Proteomics Assay Chip: Lung Cancer Cell Line Case Study
Authors: Y Jung, M Son, YR Nam, J Choi, JR Heath, S Yang
Micromachines, 2021-09-23;12(10):.
Species: Human
Sample Types: Cell Lysates
-
Pulmonary function changes in older adults with and without metabolic syndrome
Authors: MAR Brandao-Ra, R Moraes-Fer, MC Oliveira-J, A Santos-Dia, ALL Bachi, G Gabriela-P, S de Oliveir, AC Araújo-Ros, LVF Oliveira, CR Frison, WL do Prado, RP Raju, PB Balagopal, RP Vieira
Scientific Reports, 2021-08-30;11(1):17337.
Species: Human
Sample Types: Exhaled Breath Condensate (EBC)
-
Evaluation of beta-Catenin Inhibition of Axitinib and Nitazoxanide in Human Monocyte-Derived Dendritic Cells
Authors: W Azeem, RM Bakke, B Gabriel, S Appel, AM Øyan, KH Kalland
Biomedicines, 2021-08-03;9(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
An In Vitro System to Study the Effect of Subchondral Bone Health on Articular Cartilage Repair in Humans
Authors: T Hopkins, KT Wright, NJ Kuiper, S Roberts, P Jermin, P Gallacher, JH Kuiper
Cells, 2021-07-27;10(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
PD-L1 recruits phospholipase C and enhances tumorigenicity of lung tumors harboring mutant forms of EGFR
Authors: S Ghosh, NB Nataraj, A Noronha, S Patkar, A Sekar, S Mukherjee, S Winograd-K, L Kramarski, A Verma, M Lindzen, DD Garcia, J Green, G Eisenberg, H Gil-Henn, A Basu, Y Lender, S Weiss, M Oren, M Lotem, B Geiger, E Ruppin, Y Yarden
Cell Reports, 2021-05-25;35(8):109181.
Species: Human
Sample Types: Cell Culture Supernates
-
HMGB1 downregulation in retinal pigment epithelial cells protects against diabetic retinopathy through the autophagy-lysosome pathway
Authors: L Feng, L Liang, S Zhang, J Yang, Y Yue, X Zhang
Autophagy, 2021-05-24;0(0):1-20.
Species: Rat
Sample Types: Serum
-
VEGF counteracts amyloid-&beta-induced synaptic dysfunction
Authors: L Martin, P Bouvet, N Chounlamou, C Watrin, R Besançon, D Pinatel, D Meyronet, J Honnorat, A Buisson, PA Salin, C Meissirel
Cell Reports, 2021-05-11;35(6):109121.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasma markers in pulmonary hypertension subgroups correlate with patient survival
Authors: T Koudstaal, D van Uden, JAC van Hulst, P Heukels, IM Bergen, LW Geenen, VJM Baggen, AE van den Bo, LM van den To, PP Chandoesin, M Kool, E Boersma, RW Hendriks, KA Boomars
Respiratory Research, 2021-05-04;22(1):137.
Species: Human
Sample Types: Plasma
-
Targeting autocrine amphiregulin robustly and reproducibly inhibits ovarian cancer in a syngeneic model: roles for wildtype p53
Authors: M Lindzen, S Ghosh, A Noronha, D Drago, NB Nataraj, O Leitner, S Carvalho, E Zmora, S Sapoznik, KB Shany, K Levanon, D Aderka, BS Ramírez, M Dahlhoff, I McNeish, Y Yarden
Oncogene, 2021-04-30;0(0):.
Species: Human
Sample Types: Ascites Fluid
-
Transcriptional Regulation of Thrombin-Induced Endothelial VEGF Induction and Proangiogenic Response
Authors: R Catar, G Moll, I Hosp, M Simon, C Luecht, H Zhao, D Wu, L Chen, J Kamhieh-Mi, K Korybalska, D Zickler, J Witowski
Cells, 2021-04-15;10(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Sinularin, an Anti-Cancer Agent Causing Mitochondria-Modulated Apoptosis and Cytoskeleton Disruption in Human Hepatocellular Carcinoma
Authors: CY Ko, PC Shih, PW Huang, YH Lee, YF Chen, MH Tai, CH Liu, ZH Wen, HM Kuo
International Journal of Molecular Sciences, 2021-04-11;22(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Melanoma Associated Chitinase 3-Like 1 Promoted Endothelial Cell Activation and Immune Cell Recruitment
Authors: G Ramos-Espi, Y Wang, JM Brandner, SW Schneider, C Gorzelanny
International Journal of Molecular Sciences, 2021-04-10;22(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of Bovine Colostrum with or without Egg on In Vitro Bacterial-Induced Intestinal Damage with Relevance for SIBO and Infectious Diarrhea
Authors: RJ Playford, N Choudhry, P Kelly, T Marchbank
Nutrients, 2021-03-22;13(3):.
Species: Human
Sample Types: Cell Lysates
-
TGF&beta1 induces in-vitro and ex-vivo angiogenesis through VEGF production in human ovarian follicular fluid-derived granulosa cells during in-vitro fertilization cycle
Authors: TH Lai, HT Chen, WB Wu
Journal of reproductive immunology, 2021-03-20;145(0):103311.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of resveratrol on the expression of VEGF, TGF-&beta, and MMP-9 in endometrial stromal cells of women with endometriosis
Authors: T Arablou, N Aryaeian, S Khodaverdi, R Kolahdouz-, Z Moradi, N Rashidi, AA Delbandi
Scientific Reports, 2021-03-15;11(1):6054.
Species: Human
Sample Types: Cell Culture Supernates
-
The NFIB-ERO1A axis promotes breast cancer metastatic colonization of disseminated tumour cells
Authors: F Zilli, P Marques Ra, P Auf der Ma, C Jehanno, A Sethi, MM Coissieux, T Eichlisber, L Sauteur, A Rouchon, L Bonapace, J Pinto Cout, R Rad, MR Jensen, A Banfi, MB Stadler, M Bentires-A
Embo Molecular Medicine, 2021-03-10;0(0):e13162.
Species: Human
Sample Types: Cell Culture Supernates
-
Initial Identification of UDP-Glucose Dehydrogenase as a Prognostic Marker in Breast Cancer Patients, Which Facilitates Epirubicin Resistance and Regulates Hyaluronan Synthesis in MDA-MB-231 Cells
Authors: DL Vitale, I Caon, A Parnigoni, I Sevic, FM Spinelli, A Icardi, A Passi, D Vigetti, L Alaniz
Biomolecules, 2021-02-09;11(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Influence of Melatonin and Light on VEGF Secretion in Primary RPE Cells
Authors: A Klettner, M Kampers, D Töbelmann, J Roider, M Dittmar
Biomolecules, 2021-01-16;11(1):.
Species: Porcine
Sample Types: Cell Culture Supernates
-
IL-33 and Superantigenic Activation of Human Lung Mast Cells Induce the Release of Angiogenic and Lymphangiogenic Factors
Authors: L Cristinzia, R Poto, G Criscuolo, AL Ferrara, MR Galdiero, L Modestino, S Loffredo, A de Paulis, G Marone, G Spadaro, G Varricchi
Cells, 2021-01-12;10(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Evaluation of a Brown Seaweed Extract from Dictyosiphon foeniculaceus as a Potential Therapeutic Agent for the Treatment of Glioblastoma and Uveal Melanoma
Authors: P Dörschmann, C Schmitt, KS Bittkau, S Neupane, M Synowitz, J Roider, S Alban, J Held-Feind, A Klettner
Marine Drugs, 2020-12-08;18(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Proteomics of regenerated tissue in response to a titanium implant with a bioactive surface in a rat tibial defect model
Authors: RM Boteanu, VI Suica, L Ivan, F Safciuc, E Uyy, E Dragan, SM Croitoru, V Grumezescu, M Chiritoiu, LE Sima, C Vlagioiu, G Socol, F Antohe
Sci Rep, 2020-10-28;10(1):18493.
Species: Rat
Sample Types: Cell Culture Supernates
-
Abnormal Nailfold Capillaries in Patients after Hand Transplantation
Authors: D Sikorska, W Samborski, D Kami?ska, M Kusztal, J Jab?ecki, K Nijakowski, A Oko, M Karczewski, K Korybalska, J Witowski
J Clin Med, 2020-10-25;9(11):.
Species: Human
Sample Types: Serum
-
Regional hyperthermia enhances mesenchymal stem cell recruitment to tumor stroma: Implications for mesenchymal stem cell-based tumor therapy
Authors: M Tutter, C Schug, KA Schmohl, S Urnauer, C Kitzberger, N Schwenk, M Petrini, C Zach, S Ziegler, P Bartenstei, WA Weber, G Multhoff, E Wagner, LH Lindner, PJ Nelson, C Spitzweg
Mol Ther, 2020-10-15;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of EMMPRIN/CD147 in regulating angiogenesis in patients with psoriatic arthritis
Authors: MA Rahat, M Safieh, E Simanovich, E Pasand, T Gazitt, A Haddad, M Elias, D Zisman
Arthritis Res Ther, 2020-10-14;22(1):240.
Species: Human
Sample Types: Serum
-
Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment
Authors: J Ohmes, Y Xiao, F Wang, MD Mikkelsen, TT Nguyen, H Schmidt, A Seekamp, AS Meyer, S Fuchs
Mar Drugs, 2020-09-21;18(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Ischemia-Like Stress Conditions Stimulate Trophic Activities of Adipose-Derived Stromal/Stem Cells
Authors: J Bachmann, E Ehlert, M Becker, C Otto, K Radeloff, T Blunk, P Bauer-Krei
Cells, 2020-08-21;9(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
VEGFA and NFE2L2 Gene Expression and Regulation by MicroRNAs in Thyroid Papillary Cancer and Colloid Goiter
Authors: LP Stuchi, MMU Castanhole, N Maniezzo-S, PM Biselli-Ch, T Henrique, JA Padovani N, D de-Santi N, AP Girol, EC Pavarino, EM Goloni-Ber
Genes (Basel), 2020-08-19;11(9):.
Species: Human
Sample Types: Tissue Homogenates
-
Differential expression of angiogenesis-related miRNAs and VEGFA in cirrhosis and hepatocellular carcinoma
Authors: ARCP de Oliveir, MMU Castanhole, PM Biselli-Ch, ÉC Pavarino, RCMA da Silva, RF da Silva, EM Goloni-Ber
Arch Med Sci, 2020-08-10;16(5):1150-1157.
Species: Human
Sample Types: Protein
-
Bis(maltolato)oxovanadium(IV) Induces Angiogenesis via Phosphorylation of VEGFR2
Authors: L Parma, HAB Peters, ME Johansson, S Gutiérrez, H Meijerink, S de Kimpe, MR de Vries, PHA Quax
Int J Mol Sci, 2020-06-30;21(13):.
Species: Human
Sample Types: Cell Culture Supernates
-
Mesenchymal Stromal Cell Bioreactor for Ex Vivo Reprogramming of Human Immune Cells
Authors: A Allen, N Vaninov, M Li, S Nguyen, P Igo, AW Tilles, B O'Rourke, BLK Miller, B Parekkadan, RN Barcia
Sci Rep, 2020-06-23;10(1):10142.
Species: Human
Sample Types: Cell Culture Supernates
-
Angiopoietins, Vascular Endothelial Growth Factors and Secretory Phospholipase A2 in Ischemic and Non-Ischemic Heart Failure
Authors: G Varricchi, S Loffredo, L Bencivenga, AL Ferrara, G Gambino, N Ferrara, A de Paulis, G Marone, G Rengo
J Clin Med, 2020-06-19;9(6):.
Species: Human
Sample Types: Plasma
-
miR-23a-3p is a Key Regulator of IL-17C-Induced Tumor Angiogenesis in Colorectal Cancer
Authors: Y Lee, SJ Kim, J Choo, G Heo, JW Yoo, Y Jung, SH Rhee, E Im
Cells, 2020-06-01;9(6):.
Species: Human, Xenograft
Sample Types: Cell Culture Supernates
-
Efficient blockade of locally reciprocated tumor-macrophage signaling using a TAM-avid nanotherapy
Authors: SJ Wang, R Li, TSC Ng, G Luthria, MJ Oudin, M Prytyskach, RH Kohler, R Weissleder, DA Lauffenbur, MA Miller
Sci Adv, 2020-05-22;6(21):eaaz8521.
Species: Human
Sample Types: Cell Lysates
-
Interleukin (IL)-17/IL-36 axis participates to the crosstalk between endothelial cells and keratinocytes during inflammatory skin responses
Authors: L Mercurio, CM Failla, L Capriotti, C Scarponi, F Facchiano, M Morelli, S Rossi, G Pagnanelli, C Albanesi, A Cavani, S Madonna
PLoS ONE, 2020-04-30;15(4):e0222969.
Species: Human
Sample Types: Cell Culture Supernates
-
A Cross-Sectional Study Evaluating the Effects of Resistance Exercise on Inflammation and Neurotrophic Factors in Elderly Women with Obesity
Authors: HT Roh, SY Cho, WY So
J Clin Med, 2020-03-20;9(3):.
Species: Human
Sample Types: Serum
-
Apelin Affects the Progression of Osteoarthritis by Regulating VEGF-Dependent Angiogenesis and miR-150-5p Expression in Human Synovial Fibroblasts
Authors: YH Wang, SJ Kuo, SC Liu, SW Wang, CH Tsai, YC Fong, CH Tang
Cells, 2020-03-02;9(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
VEGF-A165b levels are reduced in breast cancer patients at primary diagnosis but increase after completion of cancer treatment
Authors: MM Karsten, MH Beck, A Rademacher, J Knabl, JU Blohmer, J Jückstock, JC Radosa, P Jank, B Rack, W Janni
Sci Rep, 2020-02-27;10(1):3635.
Species: Human
Sample Types: Whole Blood
-
Vitamin-D3 (&alpha-1, 25(OH) 2D3) Protects Retinal Pigment Epithelium From Hyperoxic Insults
Authors: P Murugeswar, A Firoz, S Murali, A Vinekar, L Krishna, VR Anandula, N Jeyabalan, P Chevour, C Jayadev, R Shetty, G Carpentier, G Kumaramani, A Ghosh, D Das
Invest. Ophthalmol. Vis. Sci., 2020-02-07;61(2):4.
Species: Human
Sample Types: Cell Culture Superantes
-
Are the Immune Properties of Mesenchymal Stem Cells from Wharton's Jelly Maintained during Chondrogenic Differentiation?
Authors: C Voisin, G Cauchois, L Reppel, C Laroye, L Louarn, C Schenowitz, P Sonon, I Poras, V Wang, E D Carosell, N Benkirane-, P Moreau, N Rouas-Frei, D Bensoussan, C Huselstein
J Clin Med, 2020-02-04;9(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
A Cell-Free SDKP-Conjugated Self-Assembling Peptide Hydrogel Sufficient for Improvement of Myocardial Infarction
Authors: S Firoozi, S Pahlavan, MH Ghanian, S Rabbani, S Tavakol, M Barekat, S Yakhkeshi, E Mahmoudi, M Soleymani, H Baharvand
Biomolecules, 2020-01-30;10(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Vascular Endothelial Growth Factor A and VEGFR-1 Change during Preimplantation in Heifers
Authors: D Chiumia, AK Hankele, AE Groebner, K Schulke, HD Reichenbac, K Giller, V Zakhartche, S Bauersachs, SE Ulbrich
Int J Mol Sci, 2020-01-15;21(2):.
Species: Bovine
Sample Types: Tissue Homogenates
-
Short Communication: Interaction of Nerve Growth Factor (NGF) and Vascular Endothelial Growth Factor (VEGF) in Healthy Individuals
Authors: A Groh, K Jahn, M Gröschl, T Hillemache, J Kornhuber, S Bleich, H Frieling, A Heberlein
Dis. Markers, 2019-11-11;2019(0):7510315.
Species: Human
Sample Types: Serum
-
Mechanism and Consequences of The Impaired Hif-1alpha Response to Hypoxia in Human Proximal Tubular HK-2 Cells Exposed to High Glucose
Authors: C García-Pas, S Benito-Mar, V Moreno-Man, AB Fernández-, FJ Lucio-Caza
Sci Rep, 2019-11-01;9(1):15868.
Species: Human
Sample Types: Whole Cells
-
The neuroprotective activity of heat-treated human platelet lysate biomaterials manufactured from outdated pathogen-reduced (amotosalen/UVA) platelet concentrates
Authors: O Nebie, D Devos, V Vingtdeux, L Barro, JC Devedjian, A Jonneaux, ML Chou, R Bordet, L Buée, F Knutson, D Blum, T Burnouf
J. Biomed. Sci., 2019-10-31;26(1):89.
Species: Human
Sample Types: Cell Culture Supernates
-
Greater Circulating Copper Concentrations and Copper/Zinc Ratios are Associated with Lower Psychological Distress, But Not Cognitive Performance, in a Sample of Australian Older Adults
Authors: M Mravunac, EA Szymlek-Ga, R M Daly, B R Roberts, M Formica, J Gianoudis, S L O'Connel, C A Nowson, B R Cardoso
Nutrients, 2019-10-17;11(10):.
Species: Human
Sample Types: Serum
-
Effects of Sulfated Fucans from Laminaria hyperborea Regarding VEGF Secretion, Cell Viability, and Oxidative Stress and Correlation with Molecular Weight
Authors: P Dörschmann, G Kopplin, J Roider, A Klettner
Mar Drugs, 2019-09-25;17(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Visual hallucinations in Alzheimer's disease do not seem to be associated with chronic hypoperfusion of to visual processing areas V2 and V3 but may be associated with reduced cholinergic input to these areas
Authors: LI Sinclair, A Kumar, T Darreh-Sho, S Love
Alzheimers Res Ther, 2019-09-12;11(1):80.
Species: Human
Sample Types: Tissue Homogenates
-
A Model Based on the Combination of IFN-gamma, IP-10, Ferritin and 25-Hydroxyvitamin D for Discriminating Latent From Active Tuberculosis in Children
Authors: P Comella-De, R Abellana, R Villar-Her, MD Jean Coute, B Sallés Min, L Canales Al, M Narcisse, J Gautier, C Ascaso, I Latorre, J Dominguez, TM Perez-Porc
Front Microbiol, 2019-08-14;10(0):1855.
Species: Human
Sample Types: Plasma
-
Generation of Human PSC-Derived Kidney Organoids with Patterned Nephron Segments and a De Novo Vascular Network
Authors: JH Low, P Li, EGY Chew, B Zhou, K Suzuki, T Zhang, MM Lian, M Liu, E Aizawa, C Rodriguez, KSM Yong, Q Chen, JM Campistol, M Fang, CC Khor, JN Foo, JC Izpisua Be, Y Xia
Cell Stem Cell, 2019-07-11;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Development and characterization of a new inhibitor of heme oxygenase activity for cancer treatment
Authors: O Mucha, P Podkalicka, M Mikulski, S Barwacz, K Andrysiak, A Biela, M Mieczkowsk, N Kachamakov, D Ryszawy, A Bia?as, B Szel??ek, P Grudnik, E Majewska, K Michalik, K Jakubiec, M Bie?, N Witkowska, K Gluza, D Ekonomiuk, K Sitarz, M Ga??zowski, K Brzózka, G Dubin, A Józkowicz, J Dulak, A ?oboda
Arch. Biochem. Biophys., 2019-07-02;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
miR-126-3p down-regulation contributes to dabrafenib acquired resistance in melanoma by up-regulating ADAM9 and VEGF-A
Authors: S Caporali, A Amaro, L Levati, E Alvino, PM Lacal, S Mastroeni, F Ruffini, L Bonmassar, GC Antonini C, N Felli, A Carè, U Pfeffer, S D'Atri
J. Exp. Clin. Cancer Res., 2019-06-21;38(1):272.
Species: Human
Sample Types: Serum
-
A Unique Pattern of Mesothelial-Mesenchymal Transition Induced in the Normal Peritoneal Mesothelium by High-Grade Serous Ovarian Cancer
Authors: M Paku?a, P Uruski, A Niklas, A Wo?niak, D Szpurek, A Tykarski, J Miku?a-Pie, K Ksi??ek
Cancers (Basel), 2019-05-13;11(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Optimizing Measurement of Vascular Endothelial Growth Factor in Small Blood Samples of Premature Infants
Authors: CC Lopez Yoma, KT Preissner, B Lorenz, K Stieger
Sci Rep, 2019-05-01;9(1):6744.
Species: Human
Sample Types: Serum
-
Superantigenic Activation of Human Cardiac Mast Cells
Authors: G Varricchi, S Loffredo, F Borriello, A Pecoraro, F Rivellese, A Genovese, G Spadaro, G Marone
Int J Mol Sci, 2019-04-12;20(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Activation of the hypoxia pathway in breast cancer tissue and patient survival are inversely associated with tumor ascorbate levels
Authors: EJ Campbell, GU Dachs, HR Morrin, VC Davey, BA Robinson, MCM Vissers
BMC Cancer, 2019-04-03;19(1):307.
Species: Human
Sample Types: Tissue Homogenates
-
Plasma Rich in Growth Factors (PRGF) Disrupt the Blood-Brain Barrier Integrity and Elevate Amyloid Pathology in the Brains of 5XFAD Mice
Authors: QV Duong, ML Kintzing, WE Kintzing, IM Abdallah, AD Brannen, A Kaddoumi
Int J Mol Sci, 2019-03-25;20(6):.
Species: Human
Sample Types: Plasma
-
Ultrasonic Generation of Pulsatile and Sequential Therapeutic Delivery Profiles from Calcium-Crosslinked Alginate Hydrogels
Authors: T Emi, K Michaud, E Orton, G Santilli, C Linh, M O'Connell, F Issa, S Kennedy
Molecules, 2019-03-16;24(6):.
Species: Human
Sample Types: Hydrogel Fluid
-
Angiogenic Properties of Menstrual Stem Cells Are Impaired in Women with a History of Preeclampsia
Authors: M Varas-Godo, S Acuña-Gall, S Venegas-Du, C Hill, A Caceres-Ve, O Realini, LJ Monteiro, G Zavala, M Khoury, R Romero, G Rice, SE Illanes
Stem Cells Int, 2019-01-27;2019(0):1916542.
Species: Human
Sample Types: Cell Culture Supernates
-
Hepatic blood flow by perfusion computed tomography as an imaging biomarker for patients with gastric cancer
Authors: K Shuto, M Mori, C Kosugi, K Narushima, S Nakabayash, T Fujisiro, A Sato, K Hayano, H Shimizu, K Koda
Oncol Lett, 2019-01-25;17(3):3267-3276.
Species: Human
Sample Types: Serum
-
Four types of human platelet lysate, including one virally inactivated by solvent-detergent, can be used to propagate Wharton Jelly mesenchymal stromal cells
Authors: MS Chen, TJ Wang, HC Lin, B Thierry
New biotechnology, 2018-11-20;0(0):.
Species: Human
Sample Types: Platelet Lysates
-
Co-Expression of VEGF and IL-6 Family Cytokines is Associated with Decreased Survival in HER2 Negative Breast Cancer Patients: Subtype-Specific IL-6 Family Cytokine-Mediated VEGF Secretion
Authors: K Tawara, H Scott, J Emathinger, A Ide, R Fox, D Greiner, D LaJoie, D Hedeen, M Nandakumar, AJ Oler, R Holzer, C Jorcyk
Transl Oncol, 2018-11-12;12(2):245-255.
Species: Human
Sample Types: Cell Culture Supernates
-
Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response
Authors: H Rammal, C Bour, M Dubus, L Entz, L Aubert, SC Gangloff, S Audonnet, NB Bercu, F Boulmedais, C Mauprivez, H Kerdjoudj
Int J Mol Sci, 2018-11-03;19(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Tissue-engineered 3D melanoma model with blood and lymphatic capillaries for drug development
Authors: J Bourland, J Fradette, FA Auger
Sci Rep, 2018-09-04;8(1):13191.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum from patients with chronic obstructive pulmonary disease induces senescence-related phenotype in bronchial epithelial cells
Authors: B Ku?nar-Kam, J Miku?a-Pie, A Witucka, A Romaniuk, N Konieczna, B Rubi?, K Ksi??ek, A Tykarski, H Batura-Gab
Sci Rep, 2018-08-28;8(1):12940.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel oncogenic and chemoresistance-inducing functions of resistin in ovarian cancer cells require miRNAs-mediated induction of epithelial-to-mesenchymal transition
Authors: L Qiu, GF Zhang, L Yu, HY Wang, XJ Jia, TJ Wang
Sci Rep, 2018-08-21;8(1):12522.
Species: Human
Sample Types: Cell Culture Supernates
-
Transplantation of Allogeneic Pericytes Improves Myocardial Vascularization and Reduces Interstitial Fibrosis in a Swine Model of Reperfused Acute Myocardial Infarction
Authors: VV Alvino, R Fernández-, I Rodriguez-, S Slater, G Mangialard, E Avolio, H Spencer, L Culliford, S Hassan, L Sueiro Bal, A Herman, A Ayaon-Alba, C Galán-Arri, J Sánchez-Go, H Hennessey, C Delmege, R Ascione, C Emanueli, GD Angelini, B Ibanez, P Madeddu
J Am Heart Assoc, 2018-01-22;7(2):.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study
Authors: F Klatte-Sch, T Schmidt, M Uckert, S Scheffler, U Kalus, M Rojewski, H Schrezenme, A Pruss, B Wildemann
Int J Mol Sci, 2018-01-10;19(1):.
Species: Human
Sample Types: Plasma
-
ADAM9 promotes lung cancer progression through vascular remodeling by VEGFA, ANGPT2, and PLAT
Authors: CY Lin, CF Cho, ST Bai, JP Liu, TT Kuo, LJ Wang, YS Lin, CC Lin, LC Lai, TP Lu, CY Hsieh, CN Chu, DC Cheng, YP Sher
Sci Rep, 2017-11-08;7(1):15108.
Species: Human
Sample Types: Cell Culture Supernates
-
The Effect of Aquaporin 1-Inhibition on Vasculogenic Mimicry in Malignant Mesothelioma
Authors: E Pulford, J McEvoy, A Hocking, S Prabhakara, K Griggs, S Klebe
Int J Mol Sci, 2017-11-01;18(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Collagen-Based Medical Device as a Stem Cell Carrier for Regenerative Medicine
Authors: L Aubert, M Dubus, H Rammal, C Bour, C Mongaret, C Boulagnon-, R Garnotel, C Schneider, R Rahouadj, C Laurent, SC Gangloff, F Velard, C Mauprivez, H Kerdjoudj
Int J Mol Sci, 2017-10-21;18(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Resveratrol reverses the adverse effects of bevacizumab on cultured ARPE-19 cells
Authors: M Subramani, M Ponnalagu, L Krishna, N Jeyabalan, P Chevour, A Sharma, C Jayadev, R Shetty, N Begum, G Archunan, D Das
Sci Rep, 2017-09-25;7(1):12242.
Species: Human
Sample Types: Cell Culture Supernates
-
The Proangiogenic Capabilities of Malignant Ascites Generated by Aggressive Ovarian Tumors
Authors: J Miku?a-Pie, P Uruski, S Szubert, K Maksin, R Moszy?ski, D Szpurek, A Wo?niak, S Sajdak, A Tykarski, K Ksi??ek
Biomed Res Int, 2017-09-20;2017(0):2592496.
Species: Human
Sample Types: Cell Culture Supernates
-
Hypoxia-Inducible Factor-Dependent Expression of Angiopoietin-Like 4 by Conjunctival Epithelial Cells Promotes the Angiogenic Phenotype of Pterygia
Authors: Q Meng, Y Qin, M Deshpande, F Kashiwabuc, M Rodrigues, Q Lu, H Ren, JH Elisseeff, GL Semenza, SV Montaner, A Sodhi
Invest. Ophthalmol. Vis. Sci., 2017-09-01;58(11):4514-4523.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor microenvironment conditions alter Akt and Na(+)/H(+) exchanger NHE1 expression in endothelial cells more than hypoxia alone: implications for endothelial cell function in cancer
Authors: AK Pedersen, J Mendes Lop, N Mørup, K Tritsaris, SF Pedersen
BMC Cancer, 2017-08-14;17(1):542.
Species: Human
Sample Types: Cell Culture Supernates
-
Minocycline modulates NF?B phosphorylation and enhances antimicrobial activity against Staphylococcus aureus in mesenchymal stromal/stem cells
Authors: AD Guerra, WE Rose, P Hematti, WJ Kao
Stem Cell Res Ther, 2017-07-21;8(1):171.
Species: Human
Sample Types: Cell Culture Supernates
-
A combination of platelet features allows detection of early-stage cancer
Authors: S Sabrkhany, MJE Kuijpers, SMJ van Kuijk, L Sanders, S Pineda, SWM Olde Damin, AC Dingemans, AW Griffioen, MGA Oude Egbri
Eur. J. Cancer, 2017-05-17;80(0):5-13.
Species: Human
Sample Types: Platelets
-
Deletion of lactate dehydrogenase-A in myeloid cells triggers antitumor immunity
Authors: P Seth, E Csizmadia, A Hedblom, M Vuerich, H Xie, M Li, MS Longhi, B Wegiel
Cancer Res., 2017-04-26;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro
Authors: E Redondo-Ca, C Cunningham, J Miller, L Martuscell, S Aoulad-Ali, NJ Rothwell, CM Kielty, SM Allan, E Pinteaux
Stem Cell Res Ther, 2017-04-17;8(1):79.
Species: Human
Sample Types: Cell Culture Supernates
-
Methoxyluteolin Inhibits Neuropeptide-stimulated Proinflammatory Mediator Release via mTOR Activation from Human Mast Cells.
Authors: Patel A, Theoharides T
J Pharmacol Exp Ther, 2017-04-12;361(3):462-471.
Species: Human
Sample Types: Cell Culture Supernates
-
Gelatin device for the delivery of growth factors involved in endochondral ossification
Authors: LA Ahrens, D Vonwil, J Christense, VP Shastri
PLoS ONE, 2017-04-05;12(4):e0175095.
Species: Chicken, Human
Sample Types: Cell Culture Supernates, Recombinant Protein
-
Effect of 2-octylcyanoacrylate on placenta derived mesenchymal stromal cells on extracellular matrix
Authors: YJ Chen, L Lankford, S Kabagambe, Z Saenz, P Kumar, D Farmer, A Wang
Placenta, 2017-04-02;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Is vascular endothelial growth factor a useful biomarker in giant cell arteritis?
Authors: N Goodfellow, J Morlet, S Singh, A Sabokbar, A Hutchings, V Sharma, J Vaskova, S Masters, A Zarei, R Luqmani
RMD Open, 2017-03-29;3(1):e000353.
Species: Human
Sample Types: Serum
-
Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept)
Authors: K El Bagdadi, A Kubesch, X Yu, S Al-Maawi, A Orlowska, A Dias, P Booms, E Dohle, R Sader, CJ Kirkpatric, J Choukroun, S Ghanaati
Eur J Trauma Emerg Surg, 2017-03-21;0(0):.
Species: Human
Sample Types: Complex Sample Type
-
Mechanisms regulating angiogenesis underlie seasonal control of pituitary function
Authors: J Castle-Mil, DO Bates, DJ Tortonese
Proc. Natl. Acad. Sci. U.S.A, 2017-03-07;114(12):E2514-E2523.
Species: Ovine
Sample Types: Tissue Homogenates
-
Effects of Ranibizumab and Aflibercept on Human M�ller Cells and Photoreceptors under Stress Conditions
Authors: W Shen, B Yau, SR Lee, L Zhu, M Yam, MC Gillies
Int J Mol Sci, 2017-03-01;18(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential regulation of blood flow induced neovascularisation and mural cell recruitment by VEGF and angiopoietin signalling
Authors: David O Bates
J. Physiol. (Lond.), 2017-02-02;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Synergic effects of VEGF-A and SDF-1 on the angiogenic properties of endothelial progenitor cells
Authors: Gabriela Odent Grig
J Tissue Eng Regen Med, 2016-12-12;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Angiogenic proteins, placental weight and perinatal outcomes among pregnant women in Tanzania
PLoS ONE, 2016-12-09;11(12):e0167716.
Species: Human
Sample Types: Plasma
-
Delphinidin inhibits angiogenesis through the suppression of HIF-1? and VEGF expression in A549 lung cancer cells
Oncol. Rep, 2016-12-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Tuberculosis-diabetes co-morbidity is characterized by heightened systemic levels of circulating angiogenic factors.
Authors: Kumar N, Moideen K, Sivakumar S, Menon P, Viswanathan V, Kornfeld H, Babu S
J Infect, 2016-10-04;74(1):10-21.
Species: Human
Sample Types: Plasma
-
Stand-Sit Microchip for High-Throughput, Multiplexed Analysis of Single Cancer Cells
Sci Rep, 2016-09-01;6(0):32505.
Applications: ChIP -
A functional bioassay to determine the activity of anti-VEGF antibody therapy in blood of patients with cancer
Br J Cancer, 2016-08-30;0(0):.
Species: Human
Sample Types: Serum
-
EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance
Nat Commun, 2016-07-29;7(0):12329.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of prostaglandin D2 on VEGF release by nasal polyp fibroblasts
Authors: K Kanai, M Okano, T Fujiwara, S Kariya, T Haruna, R Omichi, SI Makihara, Y Hirata, K Nishizaki
Allergol Int, 2016-04-16;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulating VEGF signaling in platelet concentrates via specific VEGF sequestering
Authors: DG Belair, NN Le, WL Murphy
Biomater Sci, 2016-03-24;4(5):819-25.
Species: Human
Sample Types: Plasma
-
Circulating Angiogenic Factors as Biomarkers of Disease Severity and Bacterial Burden in Pulmonary Tuberculosis.
Authors: Kumar N, Banurekha V, Nair D, Babu S
PLoS ONE, 2016-01-04;11(1):e0146318.
Species: Human
Sample Types: Plasma
-
High-grade serous ovarian cancer cell lines exhibit heterogeneous responses to growth factor stimulation.
Authors: Bourgeois D, Kabarowski K, Porubsky V, Kreeger P
Cancer Cell Int, 2015-12-07;15(0):112.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of In Vitro Engineered Human Adipose Tissues: Relevant Adipokine Secretion and Impact of TNF-alpha.
Authors: Aubin K, Safoine M, Proulx M, Audet-Casgrain M, Côté J, Tetu F, Roy A, Fradette J
PLoS ONE, 2015-09-14;10(9):e0137612.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammatory and Angiogenic Factors at Mid-Pregnancy Are Associated with Spontaneous Preterm Birth in a Cohort of Tanzanian Women.
Authors: McDonald C, Darling A, Conroy A, Tran V, Cabrera A, Liles W, Wang M, Aboud S, Urassa W, Fawzi W, Kain K
PLoS ONE, 2015-08-06;10(8):e0134619.
Species: Human
Sample Types: Plasma
-
A method to investigate the anti-metabolic activity of anti-cancer agents on ovarian cancer cells cultured in a 96-well high throughput format.
Authors: Hogg S, Evans J, Sykes P, Chitcholtan K
J Ovarian Res, 2015-07-04;8(0):43.
Species: Human
Sample Types: Cell Culture Supernates
-
Synchronous down-modulation of miR-17 family members is an early causative event in the retinal angiogenic switch.
Authors: Nunes D, Dias-Neto E, Cardo-Vila M, Edwards J, Dobroff A, Giordano R, Mandelin J, Brentani H, Hasselgren C, Yao V, Marchio S, Pereira C, Passetti F, Calin G, Sidman R, Arap W, Pasqualini R
Proc Natl Acad Sci U S A, 2015-03-09;112(12):3770-5.
Species: Human
Sample Types: Cell Culture Supernates
-
Creating capillary networks within human engineered tissues: Impact of adipocytes and their secretory products
Authors: Kim Aubin, Caroline Vincent, Maryse Proulx, Dominique Mayrand, Julie Fradette
Acta Biomaterialia
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of nitrogen containing bisphosphonates, zoledronate and alendronate, on the production of pro-angiogenic factors by osteoblastic cells.
Authors: Ishtiaq S, Edwards S, Sankaralingam A, Evans B, Elford C, Frost M, Fogelman I, Hampson G
Cytokine, 2014-11-17;71(2):154-60.
Species: Human
Sample Types: Serum
-
The radiosensitising effect of gemcitabine and its main metabolite dFdU under low oxygen conditions is in vitro not dependent on functional HIF-1 protein.
Authors: Wouters A, Pauwels B, Burrows N, Baay M, Deschoolmeester V, Vu T, Laukens K, Meijnders P, Van Gestel D, Williams K, Van den Weyngaert D, Vermorken J, Pauwels P, Peeters M, Lardon F
BMC Cancer, 2014-08-16;14(0):594.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of VEGF-A165b in trophoblast survival.
Authors: Bills V, Hamdollah-Zadeh M, Soothill P, Harper S, Bates D
BMC Pregnancy Childbirth, 2014-08-15;14(0):278.
Species: Human
Sample Types: Cell Lysates
-
Targeting SRPK1 to control VEGF-mediated tumour angiogenesis in metastatic melanoma.
Authors: Gammons, M V, Lucas, R, Dean, R, Coupland, S E, Oltean, S, Bates, D O
Br J Cancer, 2014-07-10;111(3):477-85.
Species: Human
Sample Types: Cell Lysates
-
Circulating autoantibodies to endothelial progenitor cells: binding characteristics and association with risk factors for atherosclerosis.
Authors: George J, Matucci-Cerinic M, Bar I, Shimoni S
PLoS ONE, 2014-06-19;9(6):e97836.
Species: Human
Sample Types: Serum
-
Celastrol inhibits the HIF-1alpha pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells.
Authors: Ma J, Han L, Liang H, Mi C, Shi H, Lee J, Jin X
Oncol Rep, 2014-05-23;32(1):235-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Autocrine impact of VEGF-A on uveal melanoma cells.
Authors: Koch K, Refaian N, Hos D, Schlereth S, Bosch J, Cursiefen C, Heindl L
Invest Ophthalmol Vis Sci, 2014-04-25;55(4):2697-704.
Species: Human
Sample Types: Cell Culture Supernates
-
Benefits of whole body vibration training in patients hospitalised for COPD exacerbations - a randomized clinical trial.
Authors: Greulich T, Nell C, Koepke J, Fechtel J, Franke M, Schmeck B, Haid D, Apelt S, Filipovic S, Kenn K, Janciauskiene S, Vogelmeier C, Koczulla A
BMC Pulm Med, 2014-04-11;14(0):60.
Species: Human
Sample Types: Serum
-
hERG1 channels regulate VEGF-A secretion in human gastric cancer: clinicopathological correlations and therapeutical implications.
Authors: Crociani O, Lastraioli E, Boni L, Pillozzi S, Romoli M, D'Amico M, Stefanini M, Crescioli S, Masi A, Taddei A, Bencini L, Bernini M, Farsi M, Beghelli S, Scarpa A, Messerini L, Tomezzoli A, Vindigni C, Morgagni P, Saragoni L, Giommoni E, Gasperoni S, Di Costanzo F, Roviello F, De Manzoni G, Bechi P, Arcangeli A
Clin Cancer Res, 2014-01-21;20(6):1502-12.
Species: Human
Sample Types: Cell Culture Supernates
-
Host biomarkers distinguish dengue from leptospirosis in Colombia: a case-control study.
Authors: Conroy A, Gelvez M, Hawkes M, Rajwans N, Liles W, Villar-Centeno L, Kain K
BMC Infect Dis, 2014-01-20;14(0):35.
Species: Human
Sample Types: Serum
-
Fibroblasts derived from human pluripotent stem cells activate angiogenic responses in vitro and in vivo.
Authors: Shamis Y, Silva E, Hewitt K, Brudno Y, Levenberg S, Mooney D, Garlick J
PLoS ONE, 2013-12-30;8(12):e83755.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-27 inhibits epithelial-mesenchymal transition and angiogenic factor production in a STAT1-dominant pathway in human non-small cell lung cancer.
Authors: Kachroo P, Lee M, Zhang L, Baratelli F, Lee G, Srivastava M, Wang G, Walser T, Krysan K, Sharma S, Dubinett S, Lee J
J Exp Clin Cancer Res, 2013-11-25;32(0):97.
Species: Human
Sample Types: Cell Culture Supernates
-
Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model.
Authors: Kim, Kwang S, Lee, Hong J, An, Jin, Kim, Yun B, Ra, Jung Cha, Lim, Inja, Kim, Seung U
Cell Transplant, 2013-09-18;23(12):1585-97.
Species: Human
Sample Types: Cell Culture Supernates
-
Prognostic impact of PHIP copy number in melanoma: linkage to ulceration.
Authors: Bezrookove, Vladimir, De Semir, David, Nosrati, Mehdi, Tong, Schuyler, Wu, Clayton, Thummala, Suresh, Dar, Altaf A, Leong, Stanley, Cleaver, James E, Sagebiel, Richard, Miller, James R, Kashani-Sabet, Mohammed
J Invest Dermatol, 2013-09-04;134(3):783-90.
Species: Human
Sample Types: Cell Culture Supernates
-
Fcgamma receptor-induced soluble vascular endothelial growth factor receptor-1 (VEGFR-1) production inhibits angiogenesis and enhances efficacy of anti-tumor antibodies.
Authors: Justiniano S, Elavazhagan S, Fatehchand K, Shah P, Mehta P, Roda J, Mo X, Cheney C, Hertlein E, Eubank T, Marsh C, Muthusamy N, Butchar J, Byrd J, Tridandapani S
J Biol Chem, 2013-07-31;288(37):26800-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Thrombin-cleaved fragments of osteopontin are overexpressed in malignant glial tumors and provide a molecular niche with survival advantage.
Authors: Yamaguchi Y, Shao Z, Sharif S, Du X, Myles T, Merchant M, Harsh G, Glantz M, Recht L, Morser J, Leung L
J Biol Chem, 2012-11-30;288(5):3097-111.
Species: Human
Sample Types: CSF
-
Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi's sarcoma.
J. Exp. Med., 2012-10-01;209(11):1985-2000.
Species: Human
Sample Types: Cell Culture Supernates
-
Abnormal histone methylation is responsible for increased vascular endothelial growth factor 165a secretion from airway smooth muscle cells in asthma.
Authors: Clifford RL, John AE, Brightling CE
J. Immunol., 2012-06-11;189(2):819-31.
Species: Human
Sample Types: Cell Culture Supernates
-
Performance characteristics of combinations of host biomarkers to identify women with occult placental malaria: a case-control study from Malawi.
Authors: Conroy AL, Liles WC, Molyneux ME
PLoS ONE, 2011-12-12;6(12):e28540.
Species: Human
Sample Types: Plasma
-
Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli.
Authors: Chatterjee M, Huang Z, Zhang W, Jiang L, Hultenby K, Zhu L, Hu H, Nilsson GP, Li N
Blood, 2011-02-17;117(14):3907-11.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased levels of plasma haemoxygenase-1 in prostate cancer.
Authors: Blann AD, Balakrishnan B, Ryan P
Prostate Cancer Prostatic Dis., 2011-01-25;14(2):114-7.
Species: Human
Sample Types: Plasma
-
Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case-control study.
Authors: Conroy AL, Phiri H, Hawkes M, Glover S, Mallewa M, Seydel KB, Taylor TE, Molyneux ME, Kain KC
PLoS ONE, 2010-12-29;5(12):e15291.
Species: Human
Sample Types: Plasma
-
Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi's sarcoma.
Authors: Ma T, Jham BC, Hu J
Proc. Natl. Acad. Sci. U.S.A., 2010-07-26;107(32):14363-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Normal ranges of angiogenesis regulatory proteins in human platelets.
Authors: Peterson JE, Zurakowski D, Italiano JE
Am. J. Hematol., 2010-07-01;85(7):487-93.
Species: Human
Sample Types: Cell Lysates
-
Soluble forms of VEGF receptor-1 and -2 promote vascular maturation via mural cell recruitment.
Authors: Lorquet S, Berndt S, Blacher S, Gengoux E, Peulen O, Maquoi E, Noel A, Foidart JM, Munaut C, Pequeux C
FASEB J., 2010-05-19;24(10):3782-95.
Species: Human
Sample Types: Cell Lysates
-
Expression of soluble vascular endothelial growth factor receptor-1 in human monocyte-derived mature dendritic cells contributes to their antiangiogenic property.
Authors: Kishuku M, Nishioka Y, Abe S, Kishi J, Ogino H, Aono Y, Azuma M, Kinoshita K, Batmunkh R, Rentsenhand B, Makino H, Ranjan P, Minakuchi K, Sone S
J. Immunol., 2009-12-15;183(12):8176-85.
Species: Human
Sample Types: Cell Culture Supernates
-
The roles of Wnt signaling modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces.
Authors: Olivares-Navarrete R, Hyzy S, Wieland M, Boyan BD, Schwartz Z
Biomaterials, 2009-12-09;31(8):2015-24.
Species: Human
Sample Types: Cell Lysates
-
VEGF(121)b, a new member of the VEGF(xxx)b family of VEGF-A splice isoforms, inhibits neovascularisation and tumour growth in vivo.
Authors: Rennel ES, Varey AH, Churchill AJ, Wheatley ER, Stewart L, Mather S, Bates DO, Harper SJ
Br. J. Cancer, 2009-08-25;101(7):1183-93.
Species: Human
Sample Types: Cell Culture Supernates
-
Angiogenesis and lymphangiogenesis are downregulated in primary breast cancer.
Authors: Boneberg EM, Legler DF, Hoefer MM, Ohlschlegel C, Steininger H, Fuzesi L, Beer GM, Dupont-Lampert V, Otto F, Senn HJ, Furstenberger G
Br. J. Cancer, 2009-08-18;101(4):605-14.
Species: Human
Sample Types: Serum
-
Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages.
Authors: Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P
Int. J. Cancer, 2009-07-15;125(2):367-73.
Species: Human
Sample Types: Cell Culture Supernates
-
Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction.
Authors: Korf-Klingebiel M, Kempf T, Sauer T, Brinkmann E, Fischer P, Meyer GP, Ganser A, Drexler H, Wollert KC
Eur. Heart J., 2008-10-25;29(23):2851-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum levels of angiogenic factors and their prognostic relevance in bladder cancer.
Authors: Szarvas T, Jager T, Droste F, Becker M, Kovalszky I, Romics I, Ergun S, Rubben H
Pathol. Oncol. Res., 2008-09-20;15(2):193-201.
Species: Human
Sample Types: Serum
-
Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production.
Authors: Ahmed S, Marotte H, Kwan K, Ruth JH, Campbell PL, Rabquer BJ, Pakozdi A, Koch AE
Proc. Natl. Acad. Sci. U.S.A., 2008-09-16;105(38):14692-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Adenosine receptors in regulation of dendritic cell differentiation and function.
Authors: Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM
Blood, 2008-06-17;112(5):1822-31.
Species: Human
Sample Types: Cell Culture Supernates
-
Lipopolysaccharide-squamous cell carcinoma-monocyte interactions induce cancer-supporting factors leading to rapid STAT3 activation.
Authors: Kurago ZB, Lam-ubol A, Stetsenko A
Head Neck Pathol, 2008-03-01;2(1):1-12.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells.
Authors: Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P
Blood, 2007-09-11;110(13):4319-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Angiogenesis markers (VEGF, soluble receptor of VEGF and angiopoietin-1) in very early arthritis and their association with inflammation and joint destruction.
Authors: Clavel G, Bessis N, Lemeiter D, Fardellone P, Mejjad O, Menard JF, Pouplin S, Boumier P, Vittecoq O, Le Loet X, Boissier MC
Clin. Immunol., 2007-06-08;124(2):158-64.
Species: Human
Sample Types: Serum
-
Pretreatment serum endostatin as a prognostic indicator in metastatic gastric carcinoma.
Authors: Woo IS, Kim KA, Jeon HM, Hong SH, Rho SY, Koh SJ, Lee MA, Byun JH, Kang JH, Hong YS, Lee KS, Cho CS, Choi MG, Chung IS
Int. J. Cancer, 2006-12-15;119(12):2901-6.
Species: Human
Sample Types: Serum
-
The effects of dexamethasone and dehydroepiandrosterone (DHEA) on cytokines and receptor expression in a human osteoblastic cell line: potential steroid-sparing role for DHEA.
Authors: Harding G, Mak YT, Evans B, Cheung J, MacDonald D, Hampson G
Cytokine, 2006-12-11;36(1):57-68.
Species: Human
Sample Types: Cell Culture Supernates
-
Angiopoietin-2 levels are elevated in exudative pleural effusions.
Authors: Kalomenidis I, Kollintza A, Sigala I, Papapetropoulos A, Papiris S, Light RW, Roussos C
Chest, 2006-05-01;129(5):1259-66.
Species: Human
Sample Types: Pleural Effusion
-
The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term.
Authors: Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, Hunter AJ, Soothill PW, Read M, Donaldson LF, Harper SJ
Clin. Sci., 2006-05-01;110(5):575-85.
Species: Human
Sample Types: Tissue Homogenates
-
Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer.
Authors: Furstenberger G, von Moos R, Lucas R, Thurlimann B, Senn HJ, Hamacher J, Boneberg EM
Br. J. Cancer, 2006-02-27;94(4):524-31.
Species: Human
Sample Types: Serum
-
Vascular endothelial growth factor-D is a survival factor for human breast carcinoma cells.
Authors: Akahane M, Akahane T, Matheny SL, Shah A, Okajima E, Thorgeirsson UP
Int. J. Cancer, 2006-02-15;118(4):841-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta.
Authors: Li H, Gu B, Zhang Y, Lewis DF, Wang Y
Placenta, 2005-02-01;26(2):210-7.
Species: Human
Sample Types: Cell Culture Supernates
-
GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice.
Authors: Eubank TD, Roberts R, Galloway M, Wang Y, Cohn DE, Marsh CB
Immunity, 2004-12-01;21(6):831-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction.
Authors: Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L
Blood, 2004-09-02;105(1):199-206.
Species: Human
Sample Types: Plasma
FAQs
-
What forms of VEGF does Human VEGF DuoSet ELISA (Catalog # DY293B) detect?
The Human VEGF DuoSet ELISA, Catalog # DY293B, has been shown to recognize rhVEGF165, rhVEGF121, and rhVEGF165b. Additional information regarding our specificity testing can be found in the product datasheet.
DuoSet Ancillary Reagent Kits
Supplemental ELISA Products
Reviews for Human VEGF DuoSet ELISA
Average Rating: 4.5 (Based on 13 Reviews)
Have you used Human VEGF DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Straight forward protocol, used on human tissue homogeneates on a 384-well format with 450/550 correction.
I use this kit to measure VEGFA levels in cell culture supernatant with very clear results. The standard curve is great and redundant.
Great kit, very easy to follow!
The substrate incubation resulted in weak change of color and so longer incubation is required for human VEGF ELISA kit.
VEGF quantification was performed onto various cell lines and data obtained is presented with the chart attached