Mouse CCL24/Eotaxin-2/MPIF-2 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse CCL24/Eotaxin-2. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: CCL24/Eotaxin-2/MPIF-2
Eotaxin-2, also named myeloid progenitor inhibitory factor 2 (MPIF-2), is a member of the CC chemokine subfamily and is designated CCL24. Eotaxin-2 is constitutively expressed in the jejunum and spleen. It can also be induced in the lung by allergen challenge and IL-4. Mouse Eotaxin-2 precursor protein shares approximately 58% amino acid sequence identity with human Eotaxin-2.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse CCL24/Eotaxin-2/MPIF-2 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
36
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
The Effect of a TLR3 Agonist on Airway Allergic Inflammation and Viral Infection in Immunoproteasome-Deficient Mice
Authors: Schaunaman, N;Nichols, T;Cervantes, D;Hartsoe, P;Ferrington, DA;Chu, HW;
Viruses
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
Ym1 protein crystals promote type 2 immunity
Authors: Heyndrickx, I;Deswarte, K;Verstraete, K;Verschueren, KHG;Smole, U;Aegerter, H;Dansercoer, A;Hammad, H;Savvides, SN;Lambrecht, BN;
eLife
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice
Authors: Maggi, L;Camelo, GMA;Rocha, IC;Pereira Alves, W;Moreira, JMP;Almeida Pereira, T;Tafuri, WL;Rabelo, ÉML;Correa, A;Ecco, R;Negrão-Corrêa, DA;
International journal of molecular sciences
Species: Mouse
Sample Types: Serum
-
Effects of mesenchymal stem cell-derived nanovesicles in experimental allergic airway inflammation
Authors: E Bandeira, SC Jang, C Lässer, K Johansson, M Rådinger, KS Park
Respiratory Research, 2023-01-05;24(1):3.
Species: Mouse
Sample Types: BALF
-
Fucoxanthin Ameliorates Oxidative Stress and Airway Inflammation in Tracheal Epithelial Cells and Asthmatic Mice
Authors: SJ Wu, CJ Liou, YL Chen, SC Cheng, WC Huang
Cells, 2021-05-25;10(6):.
Species: Mouse
Sample Types: BALF
-
IL-33 Mediates Lung Inflammation by the IL-6-Type Cytokine Oncostatin M
Authors: F Botelho, A Dubey, EA Ayaub, R Park, A Yip, A Humbles, R Kolbeck, CD Richards
Mediators of Inflammation, 2020-11-28;2020(0):4087315.
Species: Mouse
Sample Types: BALF
-
RELMalpha is Induced in Airway Epithelial Cells by Oncostatin M Without Requirement of STAT6 or IL-6 in Mouse Lungs In Vivo
Authors: L Ho, A Yip, F Lao, F Botelho, CD Richards
Cells, 2020-05-27;9(6):.
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
House Dust Mite Induces Bone Marrow IL-33-Responsive ILC2s and TH Cells
Authors: E Boberg, K Johansson, C Malmhäll, J Weidner, M Rådinger
Int J Mol Sci, 2020-05-26;21(11):.
Species: Mouse
Sample Types: BALF
-
The Supernatant of Tonsil-Derived Mesenchymal Stem Cell Has Antiallergic Effects in Allergic Rhinitis Mouse Model
Authors: IS Park, JH Kim, JS Bae, DK Kim, JH Mo
Mediators Inflamm., 2020-04-07;2020(0):6982438.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Allergic inflammation is initiated by IL-33-dependent crosstalk between mast cells and basophils
Authors: CL Hsu, KD Chhiba, R Krier-Burr, S Hosakoppal, S Berdnikovs, ML Miller, PJ Bryce
PLoS ONE, 2020-01-15;15(1):e0226701.
Species: Mouse
Sample Types: Cell Culture Supern
-
Protective Effects of Licochalcone A Improve Airway Hyper-Responsiveness and Oxidative Stress in a Mouse Model of Asthma
Authors: WC Huang, CY Liu, SC Shen, LC Chen, KW Yeh, SH Liu, CJ Liou
Cells, 2019-06-20;8(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Glucagon reduces airway hyperreactivity, inflammation, and remodeling induced by ovalbumin
Authors: DBR Insuela, CT Azevedo, DS Coutinho, NS Magalhães, MR Ferrero, TPT Ferreira, CM Cascabulho, A Henriques-, PC Olsen, BL Diaz, PMR Silva, RSB Cordeiro, MA Martins, VF Carvalho
Sci Rep, 2019-04-24;9(1):6478.
Species: Mouse
Sample Types: Tissue Homogenates
-
Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation
Authors: M Becerra-Dí, AB Strickland, A Keselman, NM Heller
J. Immunol., 2018-10-10;0(0):.
Species: Mouse
Sample Types: BALF
-
Eosinophils support adipocyte maturation and promote glucose tolerance in obesity
Authors: EH Lee, M Itan, J Jang, HJ Gu, P Rozenberg, MK Mingler, T Wen, J Yoon, SY Park, JY Roh, CS Choi, WJ Park, A Munitz, Y Jung
Sci Rep, 2018-07-02;8(1):9894.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Knob protein enhances epithelial barrier integrity and attenuates airway inflammation
Authors: SG Ha, M Dileepan, XN Ge, BN Kang, YG Greenberg, A Rao, G Muralidhar, L Medina-Kau, MA Thompson, CM Pabelick, SM O'Grady, SP Rao, P Sriramarao
J. Allergy Clin. Immunol., 2018-03-06;0(0):.
Species: Mouse
Sample Types: BALF
Applications: ELISA Capture -
Toll-Interacting Protein, Tollip, Inhibits IL-13-Mediated Pulmonary Eosinophilic Inflammation in Mice
Authors: Y Ito, N Schaefer, A Sanchez, D Francisco, R Alam, RJ Martin, JG Ledford, C Stevenson, D Jiang, L Li, M Kraft, HW Chu
J Innate Immun, 2018-01-27;0(0):.
Species: Mouse
Sample Types: BALF
-
Eosinophil-derived CCL-6 impairs hematopoietic stem cell homeostasis
Authors: C Zhang, W Yi, F Li, X Du, H Wang, P Wu, C Peng, M Luo, W Hua, CC Wong, JJ Lee, W Li, Z Chen, S Ying, Z Ju, H Shen
Cell Res., 2018-01-12;0(0):.
Species: Mouse
Sample Types: BALF
-
Regulatory effects of Interleukin (IL)-15 on allergen-induced airway obstruction
Authors: SU Venkatesha, X Zhu, P Rajavelu, R Niranjan, M Manohar, AK Verma, JA Lasky, A Mishra
J. Allergy Clin. Immunol., 2017-06-09;0(0):.
Species: Mouse
Sample Types: BALF
-
Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells
Authors: C Li, I Maillet, C Mackowiak, C Viala, F Di Padova, M Li, D Togbe, V Quesniaux, Y Lai, B Ryffel
Cell Death Dis, 2017-04-06;8(4):e2735.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-1R1-MyD88 axis elicits papain-induced lung inflammation
Eur J Immunol, 2016-09-22;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Regulation of eosinophilia and allergic airway inflammation by the glycan-binding protein galectin-1
Proc Natl Acad Sci USA, 2016-07-25;0(0):.
Species: Mouse
Sample Types: BALF
-
Potential of PEGylated Toll-Like Receptor 7 Ligands for Controlling Inflammation and Functional Changes in Mouse Models of Asthma and Silicosis
Authors: Tatiana Paula Teixeira Ferreira, Lívia Lacerda Mariano, Roberta Ghilosso-Bortolini, Ana Carolina Santos de de Arantes, Andrey Junior Fernandes, Michelle Berni et al.
Frontiers in Immunology
Species: Mouse
Sample Types: Tissue Homogenates
-
B cells play key roles in th2-type airway immune responses in mice exposed to natural airborne allergens.
Authors: Drake L, Iijima K, Hara K, Kobayashi T, Kephart G, Kita H
PLoS ONE, 2015-03-24;10(3):e0121660.
Species: Mouse
Sample Types: BALF
-
Dendritic cells induce Th2-mediated airway inflammatory responses to house dust mite via DNA-dependent protein kinase.
Authors: Mishra, Amarjit, Brown, Alexandr, Yao, Xianglan, Yang, Shutong, Park, Sung-Jun, Liu, Chengyu, Dagur, Pradeep, McCoy, J Philip, Keeran, Karen J, Nugent, Gayle Z, Jeffries, Kenneth, Qu, Xuan, Yu, Zu-Xi, Levine, Stewart, Chung, Jay H
Nat Commun, 2015-02-18;6(0):6224.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mycoplasma pneumoniae CARDS toxin exacerbates ovalbumin-induced asthma-like inflammation in BALB/c mice.
Authors: Medina, Jorge L, Coalson, Jacqueli, Brooks, Edward G, Le Saux, Claude J, Winter, Vicki T, Chaparro, Adriana, Principe, Molly F, Solis, Laura, Kannan, T R, Baseman, Joel B, Dube, Peter H
PLoS ONE, 2014-07-24;9(7):e102613.
Species: Mouse
Sample Types: BALF
-
IRF-3, IRF-7, and IPS-1 promote host defense against acute human metapneumovirus infection in neonatal mice.
Authors: Spann K, Loh Z, Lynch J, Ullah A, Zhang V, Baturcam E, Werder R, Khajornjiraphan N, Rudd P, Loo Y, Suhrbier A, Gale M, Upham J, Phipps S
Am J Pathol, 2014-04-13;184(6):1795-806.
Species: Mouse
Sample Types: Cell Lysates
-
MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung.
Authors: Malmhall C, Alawieh S, Lu Y, Sjostrand M, Bossios A, Eldh M, Radinger M
J Allergy Clin Immunol, 2013-12-24;133(5):1429-38, 1438.
Species: Mouse
Sample Types: Tissue Homogenates
-
Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation.
Authors: Kiwamoto T, Brummet M, Wu F, Motari M, Smith D, Schnaar R, Zhu Z, Bochner B
J Allergy Clin Immunol, 2013-07-02;133(1):240-7.e1-3.
Species: Mouse
Sample Types: BALF
-
Peptidoglycan recognition protein 1 enhances experimental asthma by promoting Th2 and Th17 and limiting regulatory T cell and plasmacytoid dendritic cell responses.
Authors: Park S, Jing X, Gupta D, Dziarski R
J Immunol, 2013-02-18;190(7):3480-92.
Species: Mouse
Sample Types: BALF
-
Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner.
Authors: Kottyan LC, Collier AR, Cao KH, Niese KA, Hedgebeth M, Radu CG, Witte ON, Khurana Hershey GK, Rothenberg ME, Zimmermann N
Blood, 2009-07-29;114(13):2774-82.
Species: Mouse
Sample Types: Cell Lysates
-
CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation.
Authors: Medoff BD, Seung E, Hong S, Thomas SY, Sandall BP, Duffield JS, Kuperman DA, Erle DJ, Luster AD
J. Immunol., 2009-01-01;182(1):623-35.
Species: Mouse
Sample Types: BALF
-
Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis.
Authors: Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME
Proc. Natl. Acad. Sci. U.S.A., 2008-05-14;105(20):7240-5.
Species: Mouse
Sample Types: BALF
-
Surfactant protein D alters allergic lung responses in mice and human subjects.
Authors: Brandt EB, Mingler MK, Stevenson MD, Wang N, Khurana Hershey GK, Whitsett JA, Rothenberg ME
J. Allergy Clin. Immunol., 2008-03-19;121(5):1140-1147.e2.
Species: Mouse
Sample Types: BALF
-
Regulation of allergen-induced bone marrow eosinophilopoiesis: role of CD4+ and CD8+ T cells.
Authors: Radinger M, Bossios A, Alm AS, Jeurink P, Lu Y, Malmhall C, Sjostrand M, Lotvall J
Allergy, 2007-12-01;62(12):1410-8.
Species: Mouse
Sample Types: BALF
-
Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma.
Authors: Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O'Neill KR, O'Neill KR, Colbert DC, Colby TV, Shen H, Blackburn MR, Irvin CC, Lee JJ, Lee NA
J. Immunol., 2007-06-15;178(12):7879-89.
Species: Mouse
Sample Types: BALF
-
Persistent effects induced by IL-13 in the lung.
Authors: Fulkerson PC, Fischetti CA, Hassman LM, Nikolaidis NM, Rothenberg ME
Am. J. Respir. Cell Mol. Biol., 2006-04-27;35(3):337-46.
Species: Mouse
Sample Types: BALF
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse CCL24/Eotaxin-2/MPIF-2 DuoSet ELISA
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Mouse CCL24/Eotaxin-2/MPIF-2 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
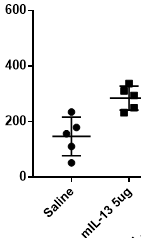
Elevated expression in mouse serum upon IL-13 stimulation