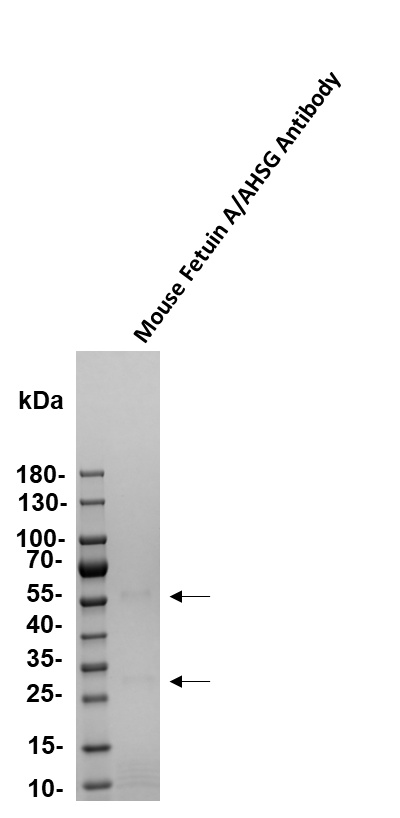
Mouse Fetuin A/AHSG Antibody Summary
Ala19-Ile345
Accession # NP_038493
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
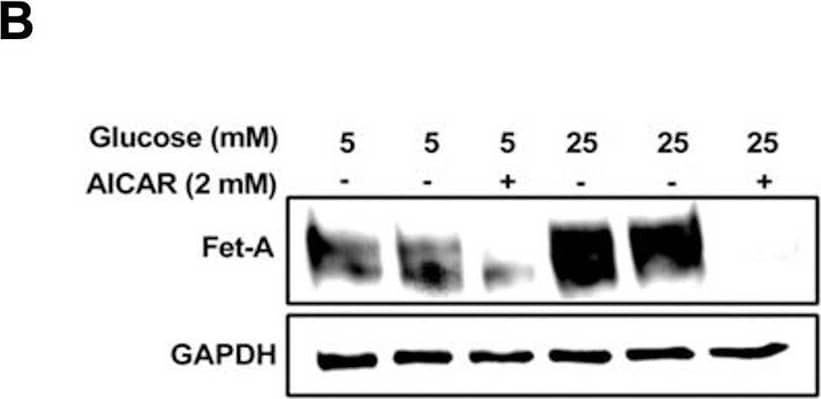
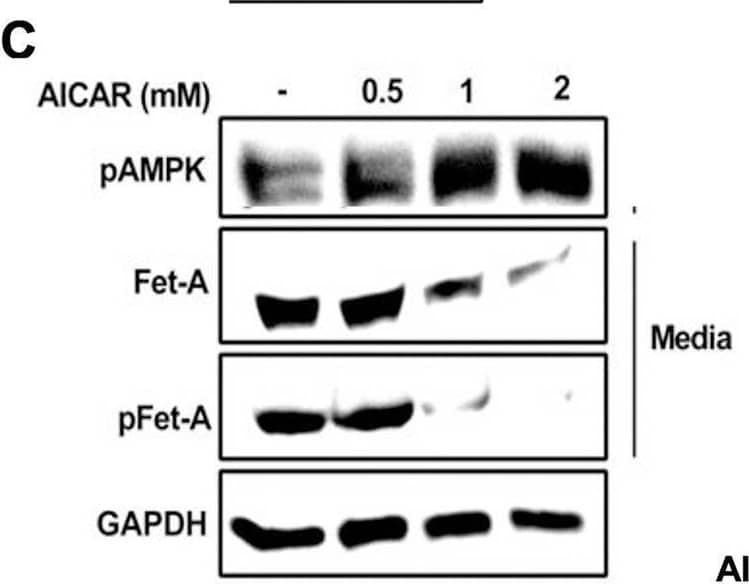
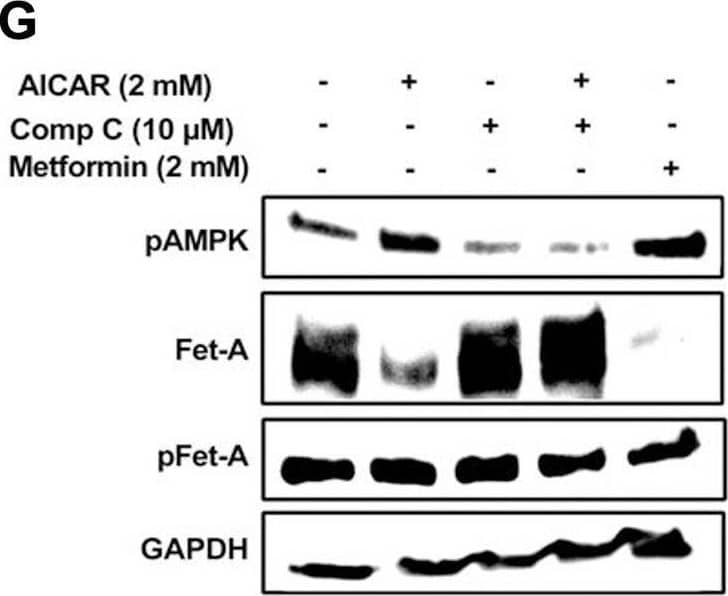
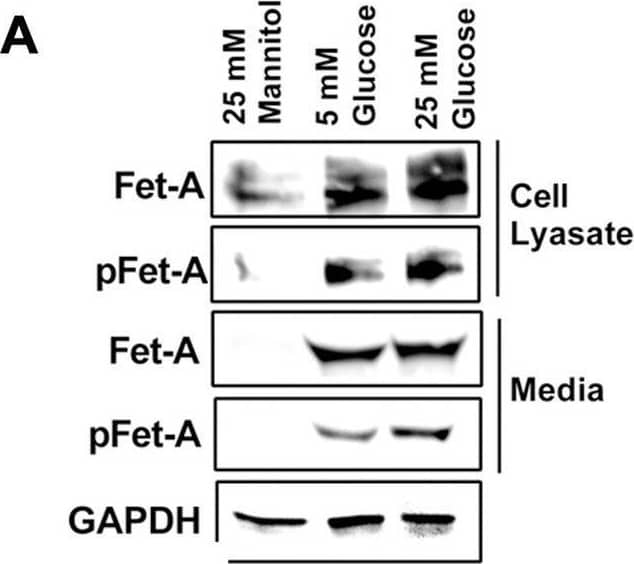
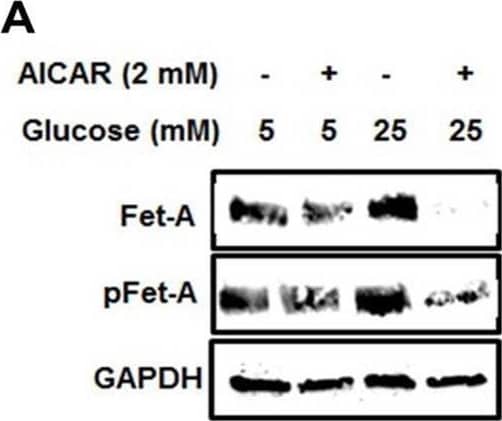
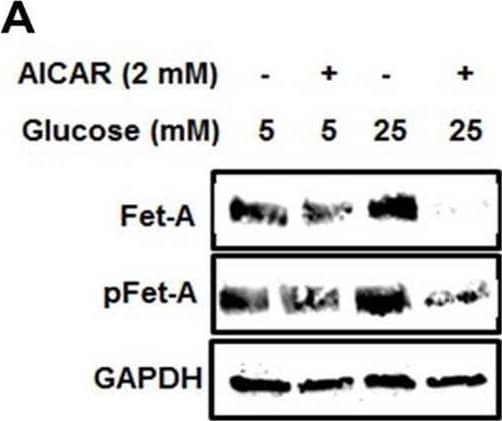
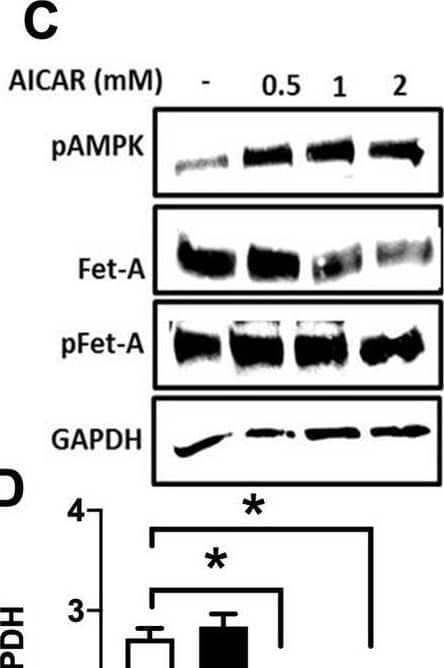
Detection of Fetuin A/AHSG by Western Blot Activation of AMPK downregulates high glucose-induced Fet-A expression in HepG2 cells.[A] HepG2 cells were incubated in a media containing either mannitol, low glucose or high glucose for 12 hours, and cell lysates/media were subjected to immunoblotting for Fet-A and pFet-A (n = 3). [B] HepG2 cells were incubated with either low- or high-glucose in the absence or presence of AICAR for 12 h and cell lysates were analyzed by Western blotting. The blots were analyzed with antibodies against Fet-A (n = 4). [C] HepG2 cells were incubated with increasing concentrations (0.5, 1, 2 mM) of AICAR for 12 h. Cell lysate or media were analyzed by Western blotting for indicated proteins (n = 3) and [D] level of Fet-A and pFet-A in media, as a ratio of GAPDH were expressed. [E] HepG2 cells were incubated in low or high glucose in the absence or presence of AICAR/metformin for 12 hours, and media was used to detect Fet-A by ELISA technique (n = 4). [F] Real-time gene expression analysis was carried out for Fet-A after AICAR and metformin treatment (n = 4). [G] HepG2 cells were incubated with AICAR/metformin in the presence or absence of Compound C, an AMPK inhibitor. Cell lysates were immunoblotted for pAMPK, Fet-A as well as p-Fet-A and [H] Fet-A levels, as a ratio to GAPDH are depicted (n = 4). Data are shown as Means ± SEM. P values were determined by one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). Data are representative of at least three independent experiments performed in replicates. * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Activation of AMPK downregulates high glucose-induced Fet-A expression in HepG2 cells.[A] HepG2 cells were incubated in a media containing either mannitol, low glucose or high glucose for 12 hours, and cell lysates/media were subjected to immunoblotting for Fet-A and pFet-A (n = 3). [B] HepG2 cells were incubated with either low- or high-glucose in the absence or presence of AICAR for 12 h and cell lysates were analyzed by Western blotting. The blots were analyzed with antibodies against Fet-A (n = 4). [C] HepG2 cells were incubated with increasing concentrations (0.5, 1, 2 mM) of AICAR for 12 h. Cell lysate or media were analyzed by Western blotting for indicated proteins (n = 3) and [D] level of Fet-A and pFet-A in media, as a ratio of GAPDH were expressed. [E] HepG2 cells were incubated in low or high glucose in the absence or presence of AICAR/metformin for 12 hours, and media was used to detect Fet-A by ELISA technique (n = 4). [F] Real-time gene expression analysis was carried out for Fet-A after AICAR and metformin treatment (n = 4). [G] HepG2 cells were incubated with AICAR/metformin in the presence or absence of Compound C, an AMPK inhibitor. Cell lysates were immunoblotted for pAMPK, Fet-A as well as p-Fet-A and [H] Fet-A levels, as a ratio to GAPDH are depicted (n = 4). Data are shown as Means ± SEM. P values were determined by one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). Data are representative of at least three independent experiments performed in replicates. * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Activation of AMPK downregulates high glucose-induced Fet-A expression in HepG2 cells.[A] HepG2 cells were incubated in a media containing either mannitol, low glucose or high glucose for 12 hours, and cell lysates/media were subjected to immunoblotting for Fet-A and pFet-A (n = 3). [B] HepG2 cells were incubated with either low- or high-glucose in the absence or presence of AICAR for 12 h and cell lysates were analyzed by Western blotting. The blots were analyzed with antibodies against Fet-A (n = 4). [C] HepG2 cells were incubated with increasing concentrations (0.5, 1, 2 mM) of AICAR for 12 h. Cell lysate or media were analyzed by Western blotting for indicated proteins (n = 3) and [D] level of Fet-A and pFet-A in media, as a ratio of GAPDH were expressed. [E] HepG2 cells were incubated in low or high glucose in the absence or presence of AICAR/metformin for 12 hours, and media was used to detect Fet-A by ELISA technique (n = 4). [F] Real-time gene expression analysis was carried out for Fet-A after AICAR and metformin treatment (n = 4). [G] HepG2 cells were incubated with AICAR/metformin in the presence or absence of Compound C, an AMPK inhibitor. Cell lysates were immunoblotted for pAMPK, Fet-A as well as p-Fet-A and [H] Fet-A levels, as a ratio to GAPDH are depicted (n = 4). Data are shown as Means ± SEM. P values were determined by one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). Data are representative of at least three independent experiments performed in replicates. * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
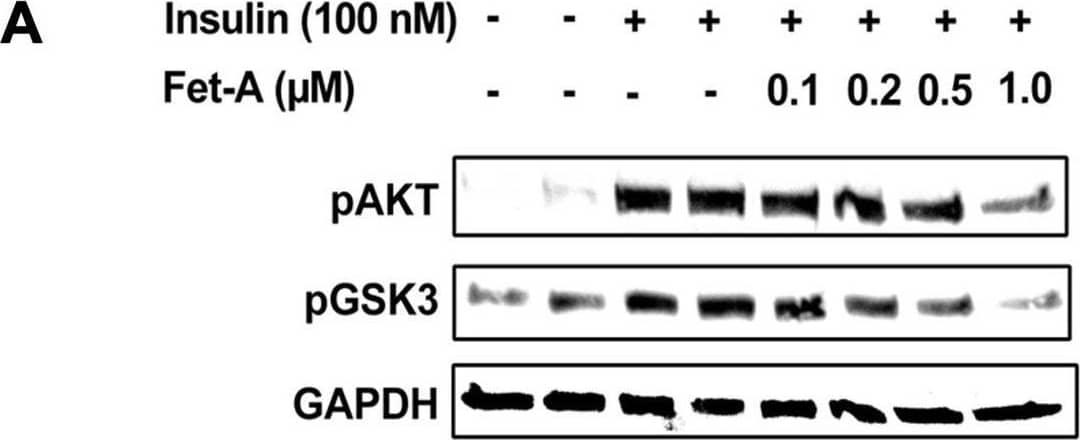
Detection of Fetuin A/AHSG by Western Blot Effect of fetuin-A (Fet-A) on insulin signaling and insulin mediated suppression of gluconeogenesis and glucose production in HepG2.[A] HepG2 cells were pre-treated with recombinant Fet-A in the presence or absence of insulin, and cell lysates were subjected to immunoblotting for AKT and GSK3 phosphorylation status (n = 3). [B] Quantified data of the ratio of cellular pAKT/GAPDH immunoblots (n = 3) and C] ratio of cellular pGSK/GAPDH immunoblots (n = 3) are shown. [D] HepG2 cells were serum-starved for 6 h, followed by treatment with dexamethasone (Dexa), insulin, or insulin and Fet-A for 12 h. Real-time gene expression of Pepck were analyzed (n = 4). [E] To analyze glucose production, HepG2 cells (n = 4) were treated with 0.5 μM dexamethasone and 0.1 mM 8-CTP-cAMP (Dex/cAMP), various concentrations of Fet-A or 100 nM insulin (Ins) in glucose free DMEM medium (pH 7.4 supplied with 20 mM sodium lactate and 2 mM sodium pyruvate) for 5 h. Glucose production was assayed by measuring glucose concentration in the medium as described previously [33]. Data are shown as Means ± SEM. P values were determined by one-way ANOVA followed by Tukey’s multiple comparison tests (A-C). Data are representative of at least three independent experiments performed in replicates. * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
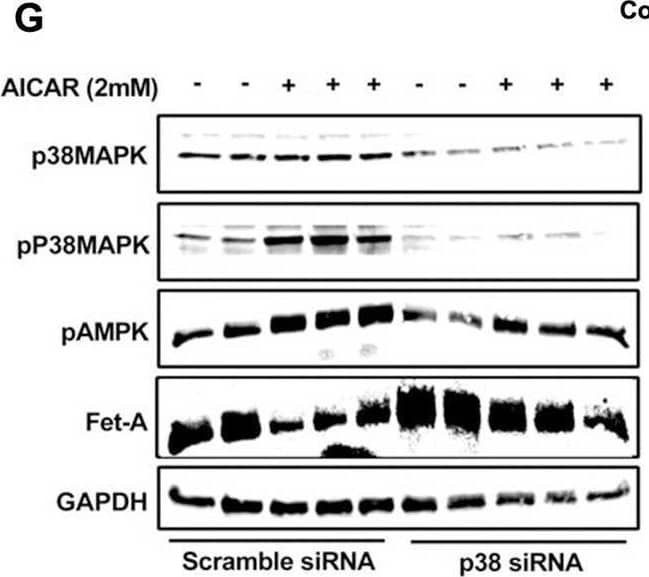
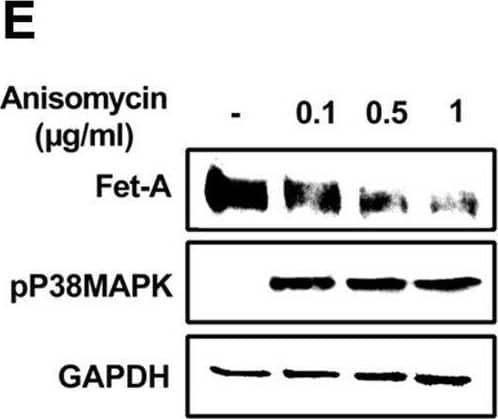
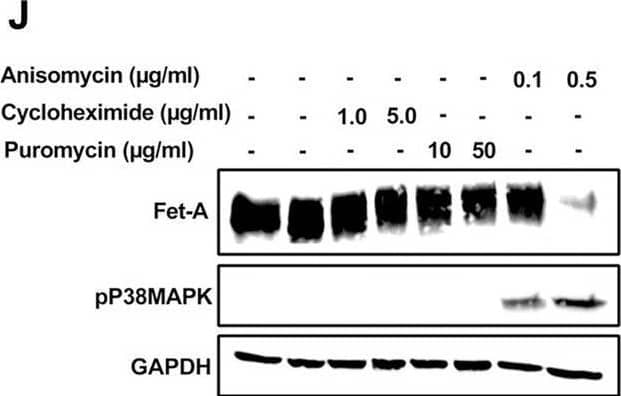
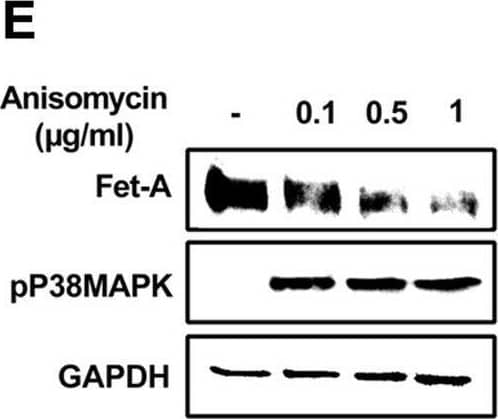
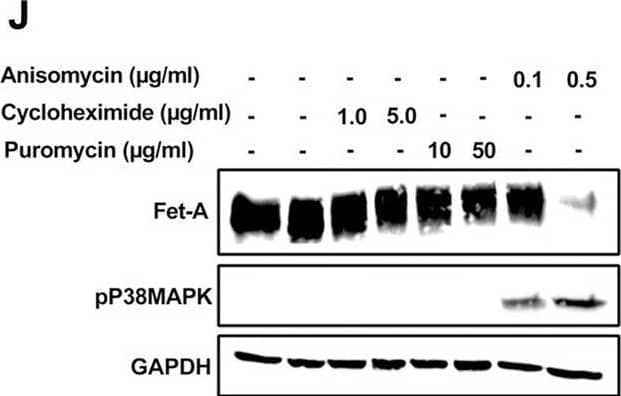
Detection of Fetuin A/AHSG by Western Blot AMPK activation downregulates Fet-A expression through p38 MAPK.[A] HepG2 were incubated with either low- or high-glucose in the absence or presence of AICAR for 12hr & used for WB analysis for ERK1/2 phosphorylation expression (n = 3). [B] HepG2 were incubated with different concentration of AICAR for 12 h & cell lysates were analyzed by WBting for ERK1/2, p38MAPK & JNK phosphorylation (n = 3). [C] were treated with p38 MAPK inhibitor [SB202190, n = 4] before treatment of AICAR for 12 hr. Cell lysate or media were analyzed by WBting for indicated proteins & [D] Fet-A levels, as a ratio to GAPDH were determined. [E] were treated with AMPK inhibitor [Comp C, n = 4] before treatment of AICAR for 12hr. Cell lysates were analyzed by WBting for phosphorylated p38 MAPK (pP38MAPK) & [F] pP38MAPK levels, as a ratio to GAPDH were determined. [G] Knockdown of p38 MAPK was performed using MAPK14 [p38 MAPK] small interfering RNA [siRNA] in HepG2. Following AICAR treatment for 12 h, cell lysates were analyzed by WBting for expression of p38 MAPK, phosphorylated p38 MAPK, Fet-A, & pAMPK. [H] Efficiency of p38MAPK siRNA in HepG2 were determined by immunoblotting transfected for p38MAPK & levels were expressed as a ratio to GAPDH. [I] Effect of AICAR on Fet-A expression in scrambled or p38MAPK siRNA transfected were determined by expressing Fet-A levels, as a ratio to GAPDH (n = 4). [J] Effect of protein synthesis inhibitors, cycloheximide & puromycin, were compared with anisomycin, also a protein synthesis inhibitor, for effects on Fet-A & phosphorylated p38 MAPK expression (n = 3). Data are shown as Means ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). * Indicates p < 0.05. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Activation of AMPK downregulates high glucose-induced Fet-A expression in HepG2 cells.[A] HepG2 cells were incubated in a media containing either mannitol, low glucose or high glucose for 12 hours, and cell lysates/media were subjected to immunoblotting for Fet-A and pFet-A (n = 3). [B] HepG2 cells were incubated with either low- or high-glucose in the absence or presence of AICAR for 12 h and cell lysates were analyzed by Western blotting. The blots were analyzed with antibodies against Fet-A (n = 4). [C] HepG2 cells were incubated with increasing concentrations (0.5, 1, 2 mM) of AICAR for 12 h. Cell lysate or media were analyzed by Western blotting for indicated proteins (n = 3) and [D] level of Fet-A and pFet-A in media, as a ratio of GAPDH were expressed. [E] HepG2 cells were incubated in low or high glucose in the absence or presence of AICAR/metformin for 12 hours, and media was used to detect Fet-A by ELISA technique (n = 4). [F] Real-time gene expression analysis was carried out for Fet-A after AICAR and metformin treatment (n = 4). [G] HepG2 cells were incubated with AICAR/metformin in the presence or absence of Compound C, an AMPK inhibitor. Cell lysates were immunoblotted for pAMPK, Fet-A as well as p-Fet-A and [H] Fet-A levels, as a ratio to GAPDH are depicted (n = 4). Data are shown as Means ± SEM. P values were determined by one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). Data are representative of at least three independent experiments performed in replicates. * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
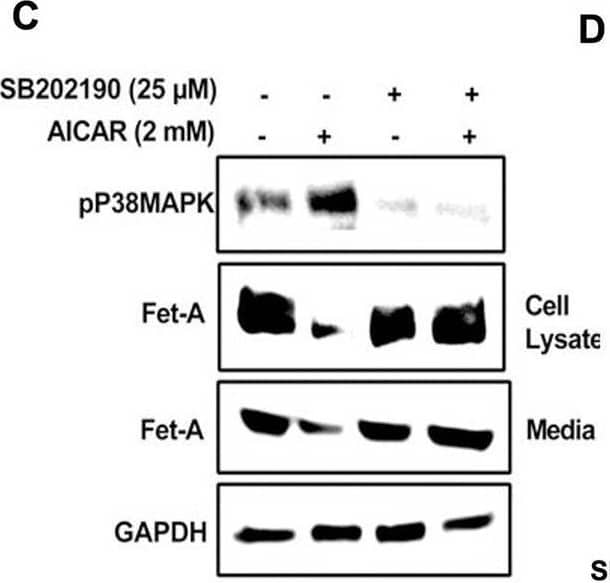 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot AMPK activation downregulates Fet-A expression through p38 MAPK.[A] HepG2 were incubated with either low- or high-glucose in the absence or presence of AICAR for 12hr & used for WB analysis for ERK1/2 phosphorylation expression (n = 3). [B] HepG2 were incubated with different concentration of AICAR for 12 h & cell lysates were analyzed by WBting for ERK1/2, p38MAPK & JNK phosphorylation (n = 3). [C] were treated with p38 MAPK inhibitor [SB202190, n = 4] before treatment of AICAR for 12 hr. Cell lysate or media were analyzed by WBting for indicated proteins & [D] Fet-A levels, as a ratio to GAPDH were determined. [E] were treated with AMPK inhibitor [Comp C, n = 4] before treatment of AICAR for 12hr. Cell lysates were analyzed by WBting for phosphorylated p38 MAPK (pP38MAPK) & [F] pP38MAPK levels, as a ratio to GAPDH were determined. [G] Knockdown of p38 MAPK was performed using MAPK14 [p38 MAPK] small interfering RNA [siRNA] in HepG2. Following AICAR treatment for 12 h, cell lysates were analyzed by WBting for expression of p38 MAPK, phosphorylated p38 MAPK, Fet-A, & pAMPK. [H] Efficiency of p38MAPK siRNA in HepG2 were determined by immunoblotting transfected for p38MAPK & levels were expressed as a ratio to GAPDH. [I] Effect of AICAR on Fet-A expression in scrambled or p38MAPK siRNA transfected were determined by expressing Fet-A levels, as a ratio to GAPDH (n = 4). [J] Effect of protein synthesis inhibitors, cycloheximide & puromycin, were compared with anisomycin, also a protein synthesis inhibitor, for effects on Fet-A & phosphorylated p38 MAPK expression (n = 3). Data are shown as Means ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). * Indicates p < 0.05. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
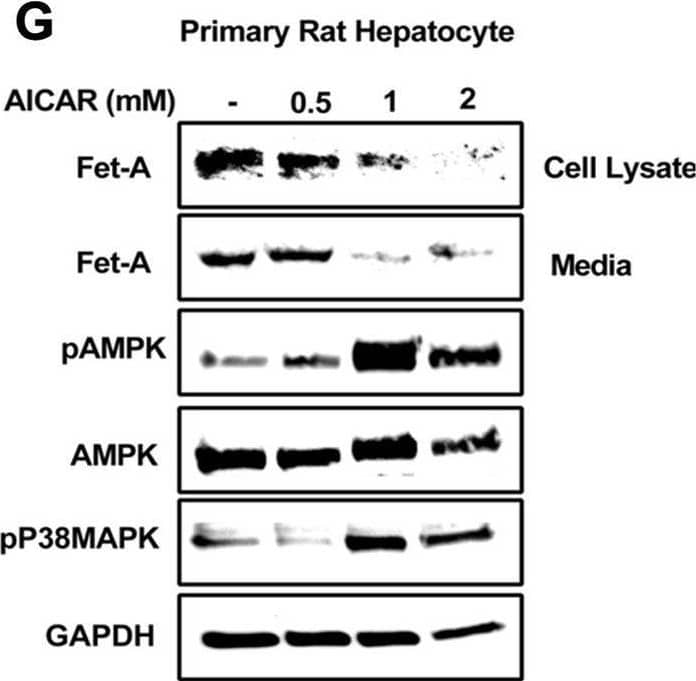
Detection of Fetuin A/AHSG by Western Blot Effect of AICAR or anisomycin treatment on Fet-A expression in Hep3B cells and primary rat hepatocytes.[A] Hep3B cells were incubated in low or high glucose media with or without AICAR for 12 hours, and cell lysates were subjected to immunoblotting for Fet-A and phosphorylated Fet-A (n = 4). [B] High glucose-mediated changes in Fet-A level in Hep3B cells was expressed as a ratio to GAPDH. [C] Hep3B cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for indicated proteins (n = 3). [D] Effect of AICAR on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH. [E] Hep3B cells were treated with various concentrations of anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK) and Fet-A expression (n = 3). [F] Effect of anisomycin on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH [G] Primary rat hepatocytes were incubated with different concentration of AICAR for 12 h and cell lysate or media were analyzed by Western blotting for indicated proteins (n = 4). [H] Effect of AICAR in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH [I] primary rat hepatocytes were treated with anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK), Fet-A and p38MAPK expression (n = 4). [J] Effect of anisomycin in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH (n = 4). Data are shown as mean ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (A-D). * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Effect of AICAR or anisomycin treatment on Fet-A expression in Hep3B cells and primary rat hepatocytes.[A] Hep3B cells were incubated in low or high glucose media with or without AICAR for 12 hours, and cell lysates were subjected to immunoblotting for Fet-A and phosphorylated Fet-A (n = 4). [B] High glucose-mediated changes in Fet-A level in Hep3B cells was expressed as a ratio to GAPDH. [C] Hep3B cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for indicated proteins (n = 3). [D] Effect of AICAR on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH. [E] Hep3B cells were treated with various concentrations of anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK) and Fet-A expression (n = 3). [F] Effect of anisomycin on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH [G] Primary rat hepatocytes were incubated with different concentration of AICAR for 12 h and cell lysate or media were analyzed by Western blotting for indicated proteins (n = 4). [H] Effect of AICAR in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH [I] primary rat hepatocytes were treated with anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK), Fet-A and p38MAPK expression (n = 4). [J] Effect of anisomycin in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH (n = 4). Data are shown as mean ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (A-D). * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot AMPK activation downregulates Fet-A expression through p38 MAPK.[A] HepG2 were incubated with either low- or high-glucose in the absence or presence of AICAR for 12hr & used for WB analysis for ERK1/2 phosphorylation expression (n = 3). [B] HepG2 were incubated with different concentration of AICAR for 12 h & cell lysates were analyzed by WBting for ERK1/2, p38MAPK & JNK phosphorylation (n = 3). [C] were treated with p38 MAPK inhibitor [SB202190, n = 4] before treatment of AICAR for 12 hr. Cell lysate or media were analyzed by WBting for indicated proteins & [D] Fet-A levels, as a ratio to GAPDH were determined. [E] were treated with AMPK inhibitor [Comp C, n = 4] before treatment of AICAR for 12hr. Cell lysates were analyzed by WBting for phosphorylated p38 MAPK (pP38MAPK) & [F] pP38MAPK levels, as a ratio to GAPDH were determined. [G] Knockdown of p38 MAPK was performed using MAPK14 [p38 MAPK] small interfering RNA [siRNA] in HepG2. Following AICAR treatment for 12 h, cell lysates were analyzed by WBting for expression of p38 MAPK, phosphorylated p38 MAPK, Fet-A, & pAMPK. [H] Efficiency of p38MAPK siRNA in HepG2 were determined by immunoblotting transfected for p38MAPK & levels were expressed as a ratio to GAPDH. [I] Effect of AICAR on Fet-A expression in scrambled or p38MAPK siRNA transfected were determined by expressing Fet-A levels, as a ratio to GAPDH (n = 4). [J] Effect of protein synthesis inhibitors, cycloheximide & puromycin, were compared with anisomycin, also a protein synthesis inhibitor, for effects on Fet-A & phosphorylated p38 MAPK expression (n = 3). Data are shown as Means ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). * Indicates p < 0.05. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Effect of AICAR or anisomycin treatment on Fet-A expression in Hep3B cells and primary rat hepatocytes.[A] Hep3B cells were incubated in low or high glucose media with or without AICAR for 12 hours, and cell lysates were subjected to immunoblotting for Fet-A and phosphorylated Fet-A (n = 4). [B] High glucose-mediated changes in Fet-A level in Hep3B cells was expressed as a ratio to GAPDH. [C] Hep3B cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for indicated proteins (n = 3). [D] Effect of AICAR on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH. [E] Hep3B cells were treated with various concentrations of anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK) and Fet-A expression (n = 3). [F] Effect of anisomycin on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH [G] Primary rat hepatocytes were incubated with different concentration of AICAR for 12 h and cell lysate or media were analyzed by Western blotting for indicated proteins (n = 4). [H] Effect of AICAR in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH [I] primary rat hepatocytes were treated with anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK), Fet-A and p38MAPK expression (n = 4). [J] Effect of anisomycin in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH (n = 4). Data are shown as mean ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (A-D). * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Effect of AICAR or anisomycin treatment on Fet-A expression in Hep3B cells and primary rat hepatocytes.[A] Hep3B cells were incubated in low or high glucose media with or without AICAR for 12 hours, and cell lysates were subjected to immunoblotting for Fet-A and phosphorylated Fet-A (n = 4). [B] High glucose-mediated changes in Fet-A level in Hep3B cells was expressed as a ratio to GAPDH. [C] Hep3B cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for indicated proteins (n = 3). [D] Effect of AICAR on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH. [E] Hep3B cells were treated with various concentrations of anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK) and Fet-A expression (n = 3). [F] Effect of anisomycin on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH [G] Primary rat hepatocytes were incubated with different concentration of AICAR for 12 h and cell lysate or media were analyzed by Western blotting for indicated proteins (n = 4). [H] Effect of AICAR in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH [I] primary rat hepatocytes were treated with anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK), Fet-A and p38MAPK expression (n = 4). [J] Effect of anisomycin in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH (n = 4). Data are shown as mean ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (A-D). * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot AMPK activation downregulates Fet-A expression through p38 MAPK.[A] HepG2 were incubated with either low- or high-glucose in the absence or presence of AICAR for 12hr & used for WB analysis for ERK1/2 phosphorylation expression (n = 3). [B] HepG2 were incubated with different concentration of AICAR for 12 h & cell lysates were analyzed by WBting for ERK1/2, p38MAPK & JNK phosphorylation (n = 3). [C] were treated with p38 MAPK inhibitor [SB202190, n = 4] before treatment of AICAR for 12 hr. Cell lysate or media were analyzed by WBting for indicated proteins & [D] Fet-A levels, as a ratio to GAPDH were determined. [E] were treated with AMPK inhibitor [Comp C, n = 4] before treatment of AICAR for 12hr. Cell lysates were analyzed by WBting for phosphorylated p38 MAPK (pP38MAPK) & [F] pP38MAPK levels, as a ratio to GAPDH were determined. [G] Knockdown of p38 MAPK was performed using MAPK14 [p38 MAPK] small interfering RNA [siRNA] in HepG2. Following AICAR treatment for 12 h, cell lysates were analyzed by WBting for expression of p38 MAPK, phosphorylated p38 MAPK, Fet-A, & pAMPK. [H] Efficiency of p38MAPK siRNA in HepG2 were determined by immunoblotting transfected for p38MAPK & levels were expressed as a ratio to GAPDH. [I] Effect of AICAR on Fet-A expression in scrambled or p38MAPK siRNA transfected were determined by expressing Fet-A levels, as a ratio to GAPDH (n = 4). [J] Effect of protein synthesis inhibitors, cycloheximide & puromycin, were compared with anisomycin, also a protein synthesis inhibitor, for effects on Fet-A & phosphorylated p38 MAPK expression (n = 3). Data are shown as Means ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (C-F). * Indicates p < 0.05. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Effect of AICAR or anisomycin treatment on Fet-A expression in Hep3B cells and primary rat hepatocytes.[A] Hep3B cells were incubated in low or high glucose media with or without AICAR for 12 hours, and cell lysates were subjected to immunoblotting for Fet-A and phosphorylated Fet-A (n = 4). [B] High glucose-mediated changes in Fet-A level in Hep3B cells was expressed as a ratio to GAPDH. [C] Hep3B cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for indicated proteins (n = 3). [D] Effect of AICAR on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH. [E] Hep3B cells were treated with various concentrations of anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK) and Fet-A expression (n = 3). [F] Effect of anisomycin on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH [G] Primary rat hepatocytes were incubated with different concentration of AICAR for 12 h and cell lysate or media were analyzed by Western blotting for indicated proteins (n = 4). [H] Effect of AICAR in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH [I] primary rat hepatocytes were treated with anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK), Fet-A and p38MAPK expression (n = 4). [J] Effect of anisomycin in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH (n = 4). Data are shown as mean ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (A-D). * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Effect of AICAR or anisomycin treatment on Fet-A expression in Hep3B cells and primary rat hepatocytes.[A] Hep3B cells were incubated in low or high glucose media with or without AICAR for 12 hours, and cell lysates were subjected to immunoblotting for Fet-A and phosphorylated Fet-A (n = 4). [B] High glucose-mediated changes in Fet-A level in Hep3B cells was expressed as a ratio to GAPDH. [C] Hep3B cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for indicated proteins (n = 3). [D] Effect of AICAR on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH. [E] Hep3B cells were treated with various concentrations of anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK) and Fet-A expression (n = 3). [F] Effect of anisomycin on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH [G] Primary rat hepatocytes were incubated with different concentration of AICAR for 12 h and cell lysate or media were analyzed by Western blotting for indicated proteins (n = 4). [H] Effect of AICAR in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH [I] primary rat hepatocytes were treated with anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK), Fet-A and p38MAPK expression (n = 4). [J] Effect of anisomycin in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH (n = 4). Data are shown as mean ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (A-D). * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
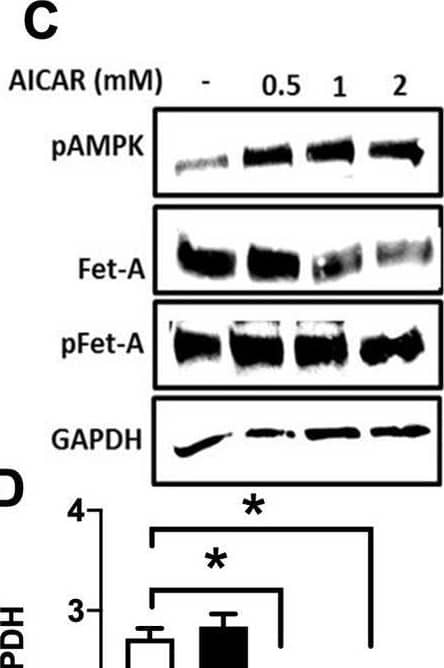 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Effect of AICAR or anisomycin treatment on Fet-A expression in Hep3B cells and primary rat hepatocytes.[A] Hep3B cells were incubated in low or high glucose media with or without AICAR for 12 hours, and cell lysates were subjected to immunoblotting for Fet-A and phosphorylated Fet-A (n = 4). [B] High glucose-mediated changes in Fet-A level in Hep3B cells was expressed as a ratio to GAPDH. [C] Hep3B cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for indicated proteins (n = 3). [D] Effect of AICAR on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH. [E] Hep3B cells were treated with various concentrations of anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK) and Fet-A expression (n = 3). [F] Effect of anisomycin on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH [G] Primary rat hepatocytes were incubated with different concentration of AICAR for 12 h and cell lysate or media were analyzed by Western blotting for indicated proteins (n = 4). [H] Effect of AICAR in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH [I] primary rat hepatocytes were treated with anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK), Fet-A and p38MAPK expression (n = 4). [J] Effect of anisomycin in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH (n = 4). Data are shown as mean ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (A-D). * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
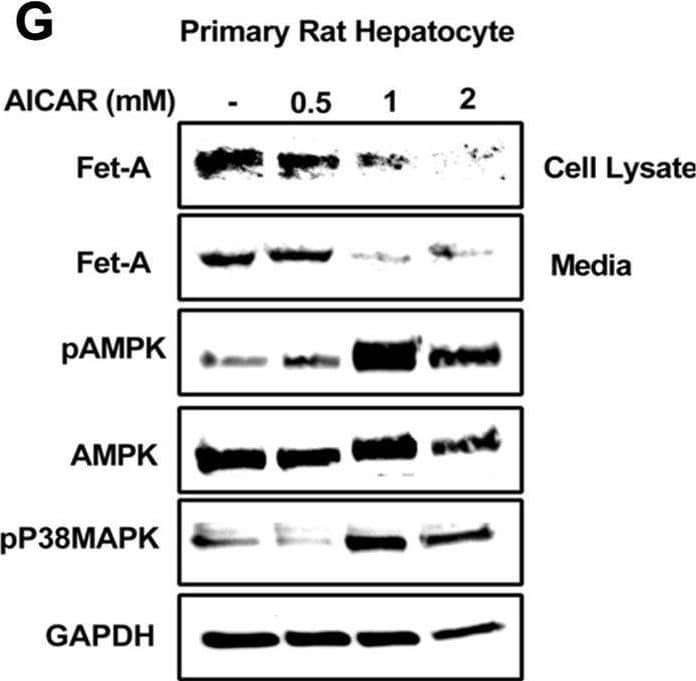 View Larger
View Larger
Detection of Fetuin A/AHSG by Western Blot Effect of AICAR or anisomycin treatment on Fet-A expression in Hep3B cells and primary rat hepatocytes.[A] Hep3B cells were incubated in low or high glucose media with or without AICAR for 12 hours, and cell lysates were subjected to immunoblotting for Fet-A and phosphorylated Fet-A (n = 4). [B] High glucose-mediated changes in Fet-A level in Hep3B cells was expressed as a ratio to GAPDH. [C] Hep3B cells were incubated with different concentration of AICAR for 12 h and cell lysates were analyzed by Western blotting for indicated proteins (n = 3). [D] Effect of AICAR on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH. [E] Hep3B cells were treated with various concentrations of anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK) and Fet-A expression (n = 3). [F] Effect of anisomycin on Fet-A level in Hep3B cells was expressed as a ratio of GAPDH [G] Primary rat hepatocytes were incubated with different concentration of AICAR for 12 h and cell lysate or media were analyzed by Western blotting for indicated proteins (n = 4). [H] Effect of AICAR in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH [I] primary rat hepatocytes were treated with anisomycin for 0.5 h to analyze phosphorylated p38 MAPK (pP38MAPK), Fet-A and p38MAPK expression (n = 4). [J] Effect of anisomycin in primary rat hepatocytes on Fet-A levels were expressed as ratio to GAPDH (n = 4). Data are shown as mean ± SEM. P values were determined accordingly by either by unpaired two-tailed test (E) or one-way ANOVA followed by Tukey’s multiple comparison tests (A-D). * Indicates p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35522655), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Fetuin A/AHSG
Mouse Fetuin A, also known as alpha 2-Heremans-Schmid glycoprotein, is encoded by the AHSG gene. It has been also called "countertrypsin" because of its ability to inhibit trypsin (1). It is a major plasma protein and a member of the cystatin superfamily of protease inhibitors (2, 3). It is expressed by hepatocytes, the principal cell source, and by monocyte/macrophages (4). The major form of plasma Fetuin A corresponds to two disulfide bond-linked chains derived from the single chain (5). Fetuin-A has a number of functions. It is a negative acute-phase protein with normal circulating levels in adults (300-600 μg/mL), which fall significantly (30-50%) during injury and infection (5). It enhances entry of cationic inhibitors into macrophages (6). It inhibits both insulin receptor autophosphorylation and undesirable calcification (7, 8).
- Yamamoto, K. and H. Sinohara (1993) J. Biol. Chem. 268:17750.
- Kellemann, J. et al. (1989) J. Biol. Chem. 264:14121.
- Dziegielewska, K.M. et al. (1990) J. Biol. Chem. 265:4354.
- Dziegielewska, K.M. et al. (1996) Histochem. Cell Biol. 106:319.
- Gejyo, F. and K. Schmid (1981) Biochim. Biophys. Acta 671:78.
- Wang, H. et al. (1998) Proc. Natl. Acad. Sci. USA 95:14429.
- Mathews, S.T. et al. (2000) Mol. Cell Endocrinol. 164:87.
- Schäfer, C. et al. (2003) J. Clin. Invest. 112:357.
Product Datasheets
Citations for Mouse Fetuin A/AHSG Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
2
Citations: Showing 1 - 2
Filter your results:
Filter by:
-
AMP activated kinase negatively regulates hepatic Fetuin-A via p38 MAPK-C/EBPbeta/E3 Ubiquitin Ligase Signaling pathway
Authors: V Kothari, JR Babu, ST Mathews
PLoS ONE, 2022-05-06;17(5):e0266472.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation, Western Blot -
Fetuin-A deficiency protects mice from Experimental Autoimmune Encephalomyelitis (EAE) and correlates with altered innate immune response
Authors: VK Harris, L Bell, RA Langan, J Tuddenham, M Landy, SA Sadiq
PLoS ONE, 2017-04-07;12(4):e0175575.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-P, Western Blot
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Fetuin A/AHSG Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Mouse Fetuin A/AHSG Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: