Mouse/Rat FGF basic/FGF2/bFGF Quantikine ELISA Kit Summary
Product Summary
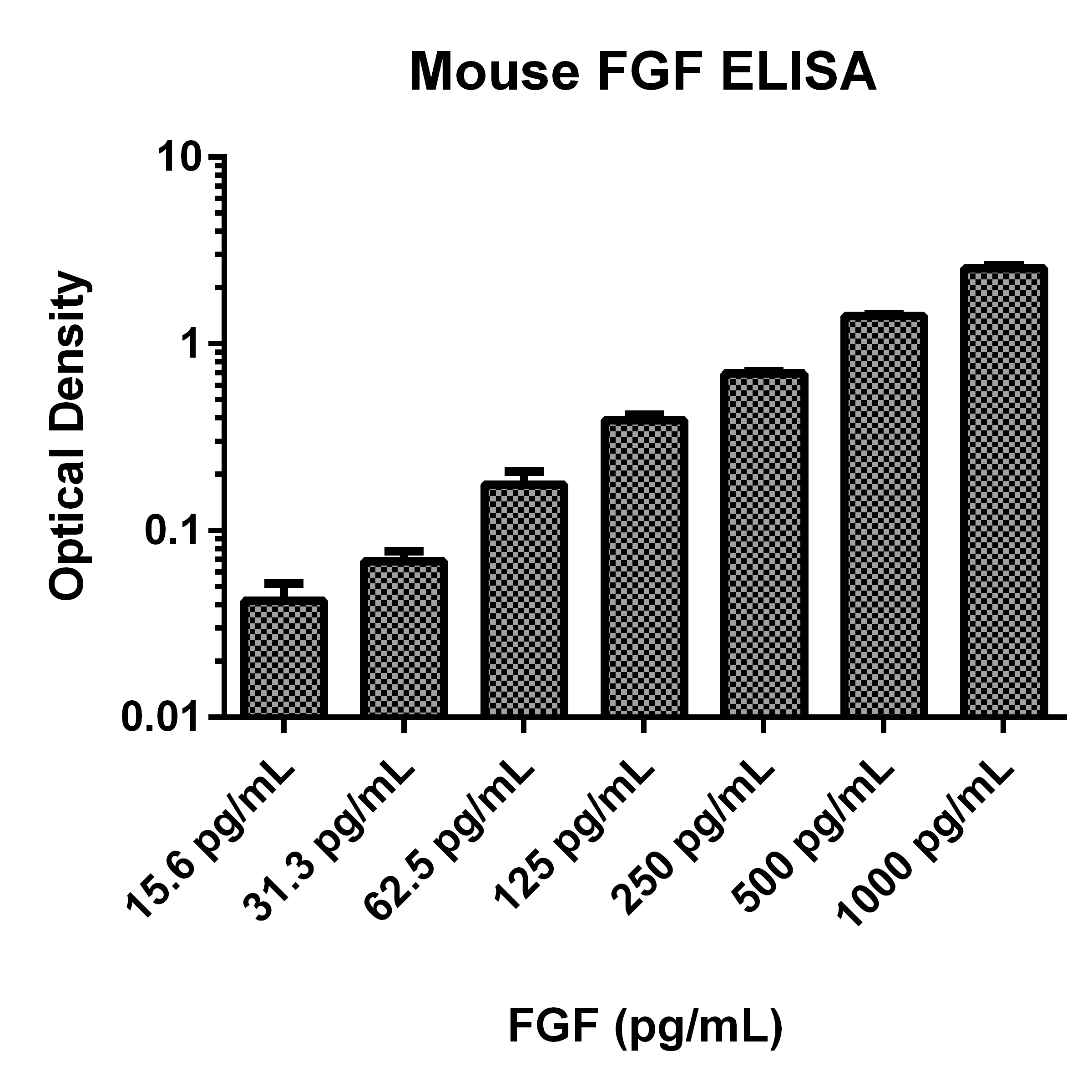
Precision
Cell Culture Supernates, Tissue Lysates, Serum, EDTA Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 49.5 | 122 | 394 | 45 | 104 | 382 |
| Standard Deviation | 1.17 | 2.7 | 12 | 3.8 | 5.03 | 20.5 |
| CV% | 2.4 | 2.2 | 3.1 | 8.4 | 4.8 | 5.4 |
Recovery
The recovery of FGF basic spiked to levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Samples (n=4) | 101 | 91-110 |
| EDTA Plasma (n=4) | 97 | 84-110 |
| Serum (n=4) | 95 | 84-110 |
| Tissue Lysates (n=4) | 97 | 80-106 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: FGF basic/FGF2/bFGF
FGF basic/FGF2/bFGF is a growth factor that functions in angiogenesis, wound healing, tissue repair, learning and memory, and the morphogenesis of heart, bone, and brain. It is upregulated in response to inflammatory stimuli and in many tumors. FGF basic/FGF2/bFGF binds to FGFR1c and 2c. Its bioactivity is modulated by a number of other binding partners including heparin, Integrin alpha V beta 3, soluble FGFR1, FGF-BP, free gangliosides, Thrombospondin, Pentraxin 3/TSG-14, Fibrinogen, alpha 2-Macroglobulin, PDGF, and CXCL4/PF4. These molecules act as cellular coreceptors or adhesion partners, extracellular matrix decoys or reservoirs, and soluble scavengers or chaperones. In particular, the interaction of FGF basic/FGF2/bFGF with cell surface heparan sulfate proteoglycans (HSPG) is required for the binding and activation of FGF receptors.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 50 µL of Assay Diluent to each well.
- Add 50 µL of Standard, Control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 100 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 4 times.
- Add 100 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 100 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Mouse/Rat FGF basic/FGF2/bFGF Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
25
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Sustained release of vascular endothelial growth factor A and basic fibroblast growth factor from nanofiber membranes reduces oxygen/glucose deprivation-induced injury to neurovascular units
Authors: Wu, Y;Sun, J;Lin, Q;Wang, D;Hai, J;
Neural regeneration research
-
Magnesium implant degradation provides immunomodulatory and proangiogenic effects and attenuates peri-implant fibrosis in soft tissues
Authors: H Ben Amara, DC Martinez, FA Shah, AJ Loo, L Emanuelsso, B Norlindh, R Willumeit-, T Plocinski, W Swieszkows, A Palmquist, O Omar, P Thomsen
Bioactive materials, 2023-03-16;26(0):353-369.
Species: Rat
Sample Types: Cell Culture Supernates
-
A simple method to isolate structurally and chemically intact brain vascular basement membrane for neural regeneration following traumatic brain injury
Authors: W Ji, Z Wu, J Wen, H Tang, Z Chen, B Xue, Z Tian, Y Ba, N Zhang, X Wen, B Hou
Biomaterials research, 2023-01-12;27(1):2.
Species: MFB00
Sample Types: Tissue Homogenates
-
Dry preserved multilayered fibroblast cell sheets are a new manageable tool for regenerative medicine to promote wound healing
Authors: Y Matsuno, M Yanagihara, K Ueno, T Saito, H Kurazumi, R Suzuki, S Katsura, A Oga, K Hamano
Scientific Reports, 2022-07-22;12(1):12519.
Species: Mouse
Sample Types: Cell Lysates
-
Enhanced cellular engraftment of adipose-derived mesenchymal stem cell spheroids by using nanosheets as scaffolds
Authors: H Nagano, Y Suematsu, M Takuma, S Aoki, A Satoh, E Takayama, M Kinoshita, Y Morimoto, S Takeoka, T Fujie, T Kiyosawa
Scientific Reports, 2021-07-14;11(1):14500.
Species: Mouse
Sample Types: Tissue Homogenates
-
Healing of Experimental Periodontal Defects Following Treatment with Fibroblast Growth Factor-2 and Deproteinized Bovine Bone Mineral
Authors: T Murakami, D Matsugami, W Yoshida, K Imamura, T Bizenjima, F Seshima, A Saito
Biomolecules, 2021-05-29;11(6):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Real-time assessment of guided bone regeneration in critical size mandibular bone defects in rats using collagen membranes with adjunct fibroblast growth factor-2
Authors: M Furuhata, T Takayama, T Yamamoto, Y Ozawa, M Senoo, M Ozaki, S Yamano, S Sato
Journal of dental sciences, 2021-04-03;16(4):1170-1181.
Species: Rat
Sample Types:
-
Bladder Regeneration Using a Polycaprolactone Scaffold with a Gradient Structure and Growth Factors in a Partially Cystectomized Rat Model
Authors: HY Kim, SY Chun, EH Lee, B Kim, YS Ha, JW Chung, JN Lee, BS Kim, SH Oh, TG Kwon
J Korean Med Sci, 2020-10-26;35(41):e374.
Species: Rat
Sample Types: Cell Culture Supernates
-
RAM-589.555 favors neuroprotective and anti-inflammatory profile of CNS-resident glial cells in acute relapse EAE affected mice
Authors: R Zilkha-Fal, T Rachutin-Z, L Cleaver, M Gurevich, A Achiron
J Neuroinflammation, 2020-10-21;17(1):313.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tyrosine kinase inhibitor neratinib attenuates liver fibrosis by targeting activated hepatic stellate cells
Authors: YJ Park, HT An, JS Park, O Park, AJ Duh, K Kim, KH Chung, KC Lee, Y Oh, S Lee
Sci Rep, 2020-09-08;10(1):14756.
Species: Mouse
Sample Types: Plasma
-
Immobilization of FGF on Poly(xylitol dodecanedioic Acid) Polymer for Tissue Regeneration
Authors: N Firoozi, Y Kang
Sci Rep, 2020-06-26;10(1):10419.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Myocardium-targeted transplantation of PHD2 shRNA-modified bone mesenchymal stem cells through ultrasound-targeted microbubble destruction protects the heart from acute myocardial infarction
Authors: Z Sun, Y Xie, RJ Lee, Y Chen, Q Jin, Q Lv, J Wang, Y Yang, Y Li, Y Cai, R Wang, Z Han, L Zhang, M Xie
Theranostics, 2020-04-06;10(11):4967-4982.
Species: Rat
Sample Types: Cell Culture Supernants
-
Cell fibers promote proliferation of co-cultured cells on a dish
Authors: A Shima, A Itou, S Takeuchi
Sci Rep, 2020-01-14;10(1):288.
Species: Mouse
Sample Types: cell culture
-
Stepwise Adipogenesis of Decellularized Cellular Extracellular Matrix Regulates Adipose Tissue-Derived Stem Cell Migration and Differentiation
Authors: Z Zhang, R Qu, T Fan, J Ouyang, F Lu, J Dai
Stem Cells Int, 2019-11-06;2019(0):1845926.
Species: Rat
Sample Types: Extracellular Matrix
-
Autophagy regulates the therapeutic potential of adipose-derived stem cells in LPS-induced pulmonary microvascular barrier damage
Authors: C Li, J Pan, L Ye, H Xu, B Wang, H Xu, L Xu, T Hou, D Zhang
Cell Death Dis, 2019-10-23;10(11):804.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mesenchymal stem cell-derived conditioned medium attenuate angiotensin II-induced aortic aneurysm growth by modulating macrophage polarization
Authors: YZ Zhou, Z Cheng, Y Wu, QY Wu, XB Liao, Y Zhao, JM Li, XM Zhou, XM Fu
J. Cell. Mol. Med., 2019-10-04;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A ZEB1/p53 signaling axis in stromal fibroblasts promotes mammary epithelial tumours
Authors: R Fu, CF Han, T Ni, L Di, LJ Liu, WC Lv, YR Bi, N Jiang, Y He, HM Li, S Wang, H Xie, BA Chen, XS Wang, SJ Weiss, T Lu, QL Guo, ZQ Wu
Nat Commun, 2019-07-19;10(1):3210.
Species: Mouse
Sample Types: Cell Lysates
-
Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression
Authors: MN Lee, HS Hwang, SH Oh, A Roshanzade, JW Kim, JH Song, ES Kim, JT Koh
Exp. Mol. Med., 2018-11-05;50(11):142.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Vascularization converts the lineage fate of bone mesenchymal stem cells to endothelial cells in tissue-engineered bone grafts by modulating FGF2-RhoA/ROCK signaling
Authors: D Li, P Cheng, H Jiang, T Cao, J Wang, Y Gao, Y Lin, C Wang, S Zhang, J Li, B Liu, Y Song, L Yang, G Pei
Cell Death Dis, 2018-09-20;9(10):959.
Species: Rat
Sample Types: Cell Culture Supernates
-
Inhibiting PHD2 in bone marrow mesenchymal stem cells via lentiviral vector-mediated RNA interference facilitates the repair of periodontal tissue defects in SD rats
Authors: C Chen, H Li, J Jiang, Q Zhang, F Yan
Oncotarget, 2017-08-14;8(42):72676-72699.
Species: Rat
Sample Types: Cell Culture Supernates
-
Fibroblast deletion of ROCK2 attenuates cardiac hypertrophy, fibrosis, and diastolic dysfunction
Authors: T Shimizu, N Narang, P Chen, B Yu, M Knapp, J Janardanan, J Blair, JK Liao
JCI Insight, 2017-07-06;2(13):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation
Proc. Natl. Acad. Sci. U.S.A, 2016-12-19;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Effect of Silodosin, an Alpha1A-Adrenoceptor Antagonist, on Ventral Prostatic Hyperplasia in the Spontaneously Hypertensive Rat.
Authors: Shimizu S, Shimizu T, Tsounapi P, Higashi Y, Martin D, Nakamura K, Honda M, Inoue K, Saito M
PLoS ONE, 2015-08-26;10(8):e0133798.
Species: Rat
Sample Types: Tissue Homogenates
-
Intra-Arterial Transplantation of Allogeneic Mesenchymal Stem Cells Mounts Neuroprotective Effects in a Transient Ischemic Stroke Model in Rats: Analyses of Therapeutic Time Window and Its Mechanisms.
Authors: Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, Sasaki T, Sasada S, Shinko A, Wakamori T, Okazaki M, Kondo A, Agari T, Borlongan C, Date I
PLoS ONE, 2015-06-15;10(6):e0127302.
Species: Rat
Sample Types: Tissue Homogenates
-
IGF2 ameliorates amyloidosis, increases cholinergic marker expression and raises BMP9 and neurotrophin levels in the hippocampus of the APPswePS1dE9 Alzheimer's disease model mice.
Authors: Mellott T, Pender S, Burke R, Langley E, Blusztajn J
PLoS ONE, 2014-04-14;9(4):e94287.
Species: Mouse
Sample Types: Tissue Homogenates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse/Rat FGF basic/FGF2/bFGF Quantikine ELISA Kit
Average Rating: 4 (Based on 1 Review)
Have you used Mouse/Rat FGF basic/FGF2/bFGF Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: