Rat VEGF Quantikine ELISA Kit Summary
Product Summary
Precision
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 65 | 60 | 65 |
| Mean (pg/mL) | 79.4 | 174 | 797 | 87.6 | 204 | 828 |
| Standard Deviation | 5.7 | 7 | 14.1 | 8.2 | 21.2 | 42.5 |
| CV% | 7.2 | 4 | 1.8 | 9.4 | 10.4 | 5.1 |
Serum, EDTA Plasma, Heparin Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 71 | 65 | 71 |
| Mean (pg/mL) | 103 | 191 | 891 | 107 | 233 | 900 |
| Standard Deviation | 3.8 | 10.7 | 19.7 | 8.5 | 23.3 | 41.1 |
| CV% | 3.7 | 5.6 | 2.2 | 7.9 | 10 | 4.6 |
Recovery
The recovery of rat VEGF spiked to three levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Supernates (n=6) | 103 | 94-108 |
| EDTA Plasma (n=6) | 102 | 95-108 |
| Heparin Plasma (n=6) | 92 | 80-100 |
| Serum (n=6) | 99 | 90-116 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: VEGF
Vascular endothelial growth factor (VEGF or VEGF-A), also known as vascular permeability factor (VPF), is a potent mediator of both angiogenesis and vasculogenesis in the fetus and in adults (1-3). It is a member of the PDGF family that is characterized by the presence of eight conserved cysteine residues in a cystine knot structure and formation of anti-parallel disulfide-linked dimers (4). Alternately spliced isoforms of 120, 164, and 188 amino acids (aa) have been found in rats and mice, while 121, 145, 165, 183, 189, and 206 aa isoforms have been identified in humans (2, 4). In humans, VEGF165 appears to be the most abundant and potent isoform, followed by VEGF121 and VEGF189 (3, 4). The same pattern may exist in rats and mice. Isoforms other than VEGF120 and VEGF121 contain basic heparin-binding regions and are not freely diffusible (4). Rat VEGF164 shares 97% aa sequence identity with corresponding regions of mouse, 88% with human and bovine, 89% with porcine and canine, and 90% with feline and equine VEGF. VEGF is expressed in multiple cells and tissues including skeletal and cardiac muscle (5, 6), hepatocytes (7), osteoblasts (8), neutrophils (9), macrophages (10), keratinocytes (11), brown adipose tissue (12), CD34+ stem cells (13), endothelial cells (14), fibroblasts, and vascular smooth muscle cells (15). VEGF expression is induced by hypoxia and cytokines such as IL-1, IL-6, IL-8, Oncostatin M, and TNF-alpha (3, 4, 9). The isoforms are differentially expressed during development and in the adult (3).
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 50 µL of Assay Diluent to each well.
- Add 50 µL of Standard, Control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours on a horizontal orbital microplate shaker.
- Aspirate each well and wash, repeating the process 4 times for a total of 5 washes.
- Add 100 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 1 hour on the shaker.
- Aspirate and wash 5 times.
- Add 100 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes on the benchtop. PROTECT FROM LIGHT.
- Add 100 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Rat VEGF Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
76
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Local low-frequency vibration accelerates healing of full-thickness wounds in a hyperglycemic rat model
Authors: Haba, D;Qin, Q;Takizawa, C;Tomida, S;Minematsu, T;Sanada, H;Nakagami, G;
Journal of diabetes investigation
Species: Rat
Sample Types: Tissue Homogenates
-
Therapeutic role of mesenchymal stem cells and platelet-rich plasma on skin burn healing and rejuvenation: A focus on scar regulation, oxido-inflammatory stress and apoptotic mechanisms
Authors: Tammam, BMH;Habotta, OA;El-Khadragy, M;Abdel Moneim, AE;Abdalla, MS;
Heliyon
Species: Rat
Sample Types: Tissue Homogenates
-
Diabetes diminishes muscle precursor cell-mediated microvascular angiogenesis
Authors: Acosta, FM;Pacelli, S;Rathbone, CR;
PloS one
Species: Rat
Sample Types: Cell Culture Supernates
-
Mesenchymal Stem/Stromal Cells in Skeletal Muscle Are Pro-Angiogenic, and the Effect Is Potentiated by Erythropoietin
Authors: Y Iso, S Usui, H Suzuki
Pharmaceutics, 2023-03-24;15(4):.
Species: Rat
Sample Types: Cell Culture Supernates
-
A Multifunctional Hybrid Nanocarrier for Non-Invasive siRNA Delivery to the Retina
Authors: S Nishida, Y Takashima, R Udagawa, H Ibaraki, Y Seta, H Ishihara
Pharmaceutics, 2023-02-11;15(2):.
Species: Rat
Sample Types: Cell Culture Supernates
-
A simple method to isolate structurally and chemically intact brain vascular basement membrane for neural regeneration following traumatic brain injury
Authors: W Ji, Z Wu, J Wen, H Tang, Z Chen, B Xue, Z Tian, Y Ba, N Zhang, X Wen, B Hou
Biomaterials research, 2023-01-12;27(1):2.
Species: Rat
Sample Types: Tissue Homogenates
-
Hydrogen sulfide attenuates lung injury instigated by Bisphenol-A via suppressing inflammation and oxidative stress
Authors: OAR Abo-Zaid, FSM Moawed, HA Hassan, EM Moustafa
Bmc Pharmacology & Toxicology, 2022-12-30;23(1):98.
Species: Rat
Sample Types: Tissue Homogenates
-
Hypoxia mimetics restore bone biomineralisation in hyperglycaemic environments
Authors: A Rezaei, Y Li, M Turmaine, S Bertazzo, CA Howard, TR Arnett, K Shakib, G Jell
Scientific Reports, 2022-08-17;12(1):13944.
Species: Rat
Sample Types: Cell Culture Supernates
-
Intratracheal Transplantation of Mesenchymal Stem Cells Attenuates Hyperoxia-Induced Microbial Dysbiosis in the Lungs, Brain, and Gut in Newborn Rats
Authors: SY Ahn, DK Sung, YS Chang, WS Park
International Journal of Molecular Sciences, 2022-06-13;23(12):.
Species: Rat
Sample Types: Tissue Homogenates
-
A novel rat model of chronic subdural hematoma: Induction of inflammation and angiogenesis in the subdural space mimicking human-like features of progressively expanding hematoma
Authors: X Xu, D Wang, Z Han, B Wang, W Gao, Y Fan, F Li, Z Zhou, C Gao, J Xiong, S Zhou, S Zhang, G Yang, R Jiang, J Zhang
Brain research bulletin, 2021-04-28;172(0):108-119.
Species: Rat
Sample Types: Tissue Homogenates
-
Probiotic-Derived Polyphosphate Accelerates Intestinal Epithelia Wound Healing through Inducing Platelet-Derived Mediators
Authors: S Isozaki, H Konishi, M Fujiya, H Tanaka, Y Murakami, S Kashima, K Ando, N Ueno, K Moriichi, T Okumura
Mediators of Inflammation, 2021-03-29;2021(0):5582943.
Species: Rat
Sample Types: Plasma
-
Comparison of steroid hormones in�three�different preeclamptic models
Authors: YY Shin, SM An, JS Jeong, SY Yang, GS Lee, EJ Hong, EB Jeung, SC Kim, BS An
Molecular Medicine Reports, 2021-02-04;23(4):.
Species: Human, Rat
Sample Types: Cell Culture Supernates, Plasma
-
Macrophage-Derived Inflammation Induces a Transcriptome Makeover in Mesenchymal Stromal Cells Enhancing Their Potential for Tissue Repair
Authors: I Maldonado-, N O'Neill, O Umland, J Verhaagen, M Oudega
International Journal of Molecular Sciences, 2021-01-14;22(2):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Optimized platelet rich plasma releasate (O-rPRP) repairs galactosemia-induced ovarian follicular loss in rats by activating mTOR signaling and inhibiting apoptosis
Authors: W El Bakly, M Medhat, M Shafei, R Tash, M Elrefai, Y Shoukry, NN Omar
Heliyon, 2020-09-21;6(9):e05006.
Species: Rat
Sample Types: Plasma
-
Combination Antioxidant/NSAID Therapies and Oral/Topical Ocular Delivery Modes for Prevention of Oxygen-Induced Retinopathy in a Rat Model
Authors: KD Beharry, CL Cai, F Siddiqui, C D'Agrosa, A Zangaladze, G Mustafa, A Qadri, TJ Duggan, JV Aranda
Nutrients, 2020-07-03;12(7):.
Species: Rat
Sample Types: Tissue Culture Supernates
-
Transplantation of Endothelial Progenitor Cells in Obese Diabetic Rats Following Myocardial Infarction: Role of Thymosin Beta-4
Authors: KK Poh, PSS Lee, AH Djohan, MJ Galupo, GG Songco, TC Yeo, HC Tan, AM Richards, L Ye
Cells, 2020-04-12;9(4):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Myocardium-targeted transplantation of PHD2 shRNA-modified bone mesenchymal stem cells through ultrasound-targeted microbubble destruction protects the heart from acute myocardial infarction
Authors: Z Sun, Y Xie, RJ Lee, Y Chen, Q Jin, Q Lv, J Wang, Y Yang, Y Li, Y Cai, R Wang, Z Han, L Zhang, M Xie
Theranostics, 2020-04-06;10(11):4967-4982.
Species: Rat
Sample Types: Cell Culture Supernants
-
Bumetanide Suppression of Angiogenesis in a Rat Model of Oxygen-Induced Retinopathy
Authors: S Guzel, CL Cai, T Ahmad, M Quan, GB Valencia, JV Aranda, KD Beharry
Int J Mol Sci, 2020-02-02;21(3):.
Species: Rat
Sample Types: Serum
-
A novel therapeutic approach using peripheral blood mononuclear cells preconditioned by oxygen-glucose deprivation
Authors: M Hatakeyama, M Kanazawa, I Ninomiya, K Omae, Y Kimura, T Takahashi, O Onodera, M Fukushima, T Shimohata
Sci Rep, 2019-11-14;9(1):16819.
Species: Human
Sample Types: Cell Culture Supernates
-
Stepwise Adipogenesis of Decellularized Cellular Extracellular Matrix Regulates Adipose Tissue-Derived Stem Cell Migration and Differentiation
Authors: Z Zhang, R Qu, T Fan, J Ouyang, F Lu, J Dai
Stem Cells Int, 2019-11-06;2019(0):1845926.
Species: Rat
Sample Types: Extracellular Matrix
-
Low-Energy Extracorporeal Shock Wave Ameliorates Streptozotocin Induced Diabetes and Promotes Pancreatic Beta Cells Regeneration in a Rat Model
Authors: CC Hsiao, CC Lin, YS Hou, JY Ko, CJ Wang
Int J Mol Sci, 2019-10-05;20(19):.
Species: Rat
Sample Types: Tissue Lysates
-
Human Umbilical Tissue-Derived Cells Secrete Soluble VEGFR1 and Inhibit Choroidal Neovascularization
Authors: J Cao, R Yang, TE Smith, S Evans, GW McCollum, SC Pomerantz, T Petley, IR Harris, JS Penn
Mol Ther Methods Clin Dev, 2019-05-22;14(0):37-46.
Species: Rat
Sample Types: Tissue Homogenates
-
Blood glutamate EAAT2-cell grabbing therapy in cerebral ischemia
Authors: M Pérez-Mato, R Iglesias-R, A Vieites-Pr, A Dopico-Lóp, B Argibay, H Fernández-, A da Silva-C, A Pérez-Díaz, C Correa-Paz, A Günther, P Ávila-Góme, M Isabel Loz, A Baumann, J Castillo, T Sobrino, F Campos
EBioMedicine, 2018-12-13;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Cell encapsulation enhances antidepressant effect of the mesenchymal stem cells and counteracts depressive-like behavior of treatment-resistant depressed rats
Authors: K Kin, T Yasuhara, M Kameda, Y Tomita, M Umakoshi, K Kuwahara, I Kin, N Kidani, J Morimoto, M Okazaki, T Sasaki, N Tajiri, CV Borlongan, I Date
Mol. Psychiatry, 2018-08-14;0(0):.
-
Radiofrequency ablation (RFA) induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins
Authors: M Ahmed, G Kumar, S Gourevitch, T Levchenko, E Galun, V Torchilin, SN Goldberg
Int J Hyperthermia, 2018-04-24;0(0):1-25.
Species: Rat
Sample Types: Serum
-
The local cytokine and growth factor response to rhBMP-2 after spinal fusion
Authors: JD Koerner, DZ Markova, GD Schroeder, BP Calio, A Shah, CW Brooks, AR Vaccaro, DG Anderson, CK Kepler
Spine J, 2018-03-14;0(0):.
Species: Rat
Sample Types: Tissue Lysates
Applications: ELISA (Standard) -
Myocardial infarction stabilization by cell-based expression of controlled Vascular Endothelial Growth Factor levels
Authors: L Melly, G Cerino, A Frobert, S Cook, MN Giraud, T Carrel, HT Tevaearai, F Eckstein, B Rondelet, A Marsano, A Banfi
J. Cell. Mol. Med., 2018-02-25;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
New arguments for beneficial effects of alpha-lipoic acid on the cardiovascular system in the course of type 2 diabetes
Authors: M Dworacka, G Chukanova, S Iskakova, Y Kurmambaye, A Weso?owska, BA Frycz, PP Jagodzi?sk, G Dworacki
Eur J Pharm Sci, 2018-02-07;117(0):41-47.
Species: Rat
Sample Types: Serum
-
?-Melanocyte-Stimulating Hormone Protects Early Diabetic Retina from Blood-Retinal Barrier Breakdown and Vascular Leakage via MC4R
Authors: S Cai, Q Yang, M Hou, Q Han, H Zhang, J Wang, C Qi, Q Bo, Y Ru, W Yang, Z Gu, R Wei, Y Cao, X Li, Y Zhang
Cell. Physiol. Biochem., 2018-01-25;45(2):505-522.
Species: Rat
Sample Types: Tissue Homogenates
-
Impact of Chronic Neonatal Intermittent Hypoxia on Severity of Retinal Damage in a Rat Model of Oxygen-Induced Retinopathy
Authors: KD Beharry, CL Cai, T Ahmad, S Guzel, GB Valencia, JV Aranda
J Nat Sci, 2018-01-01;4(3):.
Species: Rat
Sample Types: Tissue Homogenates
-
Scavenging of reactive oxygen species by astaxanthin inhibits epithelial-mesenchymal transition in high glucose-stimulated mesothelial cells
Authors: K Hara, C Hamada, K Wakabayash, R Kanda, K Kaneko, S Horikoshi, Y Tomino, Y Suzuki
PLoS ONE, 2017-09-19;12(9):e0184332.
Species: Rat
Sample Types: Cell Culture Supernates
-
Inhibiting PHD2 in bone marrow mesenchymal stem cells via lentiviral vector-mediated RNA interference facilitates the repair of periodontal tissue defects in SD rats
Authors: C Chen, H Li, J Jiang, Q Zhang, F Yan
Oncotarget, 2017-08-14;8(42):72676-72699.
Species: Rat
Sample Types: Cell Culture Supernates
-
Protective effects on the retina after ranibizumab treatment in an ischemia model
Authors: SC Joachim, M Renner, J Reinhard, C Theiss, C May, S Lohmann, S Reinehr, G Stute, A Faissner, K Marcus, HB Dick
PLoS ONE, 2017-08-11;12(8):e0182407.
Species: Rat
Sample Types: Aqueous Humor
-
Protective effect of maternal uteroplacental insufficiency on oxygen-induced retinopathy in offspring: removing bias of premature birth
Authors: S Becker, H Wang, B Yu, R Brown, X Han, RH Lane, ME Hartnett
Sci Rep, 2017-02-14;7(0):42301.
Species: Rat
Sample Types: Serum
-
Alterations in VEGF expression induced by antidepressant drugs in female rats under chronic social stress
Authors: MM Nowacka-Ch, M Paul-Samoj, AM Bielecka-W, JJ Barski, E Obuchowicz
Exp Ther Med, 2017-01-02;13(2):723-730.
Species: Rat
Sample Types: Serum
-
Mesenchymal stem cells in corneal neovascularization: Comparison of different application routes
Mol Med Rep, 2016-08-11;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Optimal Route for Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Transplantation to Protect Against Neonatal Hyperoxic Lung Injury: Gene Expression Profiles and Histopathology.
Authors: Sung D, Chang Y, Ahn S, Sung S, Yoo H, Choi S, Kim S, Park W
PLoS ONE, 2015-08-25;10(8):e0135574.
Species: Rat
Sample Types: Tissue Homogenates
-
Systemic siRNA Nanoparticle-Based Drugs Combined with Radiofrequency Ablation for Cancer Therapy.
Authors: Ahmed M, Kumar G, Navarro G, Wang Y, Gourevitch S, Moussa M, Rozenblum N, Levchenko T, Galun E, Torchilin V, Goldberg S
PLoS ONE, 2015-07-08;10(7):e0128910.
Species: Rat
Sample Types: Tissue Homogenates
-
Iron-hepcidin dysmetabolism, anemia and renal hypoxia, inflammation and fibrosis in the remnant kidney rat model.
Authors: Garrido P, Ribeiro S, Fernandes J, Vala H, Bronze-da-Rocha E, Rocha-Pereira P, Belo L, Costa E, Santos-Silva A, Reis F
PLoS ONE, 2015-04-13;10(4):e0124048.
Species: Rat
Sample Types: Serum
-
Leptin ameliorates ischemic necrosis of the femoral head in rats with obesity induced by a high-fat diet.
Authors: Zhou L, Jang K, Moon Y, Wagle S, Kim K, Lee K, Park B, Kim J
Sci Rep, 2015-03-23;5(0):9397.
Species: Rat
Sample Types: Plasma
-
Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model.
Authors: Di Pietro M, Parborell F, Irusta G, Pascuali N, Bas D, Bianchi M, Tesone M, Abramovich D
Endocrinology, 2015-01-15;156(4):1453-63.
Species: Rat
Sample Types: Cell Extracts
-
The effect of lithospermic acid, an antioxidant, on development of diabetic retinopathy in spontaneously obese diabetic rats.
Authors: Jin C, Yu S, Wang X, Woo S, Park H, Lee H, Choi S, Kim K, Kim J, Park K, Jang H, Lim S
PLoS ONE, 2014-06-06;9(6):e98232.
Species: Rat
Sample Types: Aqueous Humor
-
Antiangiogenic treatment diminishes renal injury and dysfunction via regulation of local AKT in early experimental diabetes.
Authors: Bai X, Li X, Tian J, Zhou Z
PLoS ONE, 2014-04-23;9(4):.
Species: Rat
Sample Types: Serum
-
Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential.
Authors: Klinkhammer B, Kramann R, Mallau M, Makowska A, van Roeyen C, Rong S, Buecher E, Boor P, Kovacova K, Zok S, Denecke B, Stuettgen E, Otten S, Floege J, Kunter U
PLoS ONE, 2014-03-25;9(3):e92115.
Species: Rat
Sample Types: Cell Culture Supernates
-
Targeting Muller cell-derived VEGF164 to reduce intravitreal neovascularization in the rat model of retinopathy of prematurity.
Authors: Jiang Y, Wang H, Culp D, Yang Z, Fotheringham L, Flannery J, Hammond S, Kafri T, Hartnett M
Invest Ophthalmol Vis Sci, 2014-02-10;55(2):824-31.
Species: Rat
Sample Types: Serum
-
Antagonism of bradykinin B2 receptor prevents inflammatory responses in human endothelial cells by quenching the NF-kB pathway activation.
Authors: Terzuoli E, Meini S, Cucchi P, Catalani C, Cialdai C, Maggi C, Giachetti A, Ziche M, Donnini S
PLoS ONE, 2014-01-02;9(1):e84358.
Species: Rat
Sample Types: Synovial Fluid
-
Platelet rich plasma clot releasate preconditioning induced PI3K/AKT/NFkappaB signaling enhances survival and regenerative function of rat bone marrow mesenchymal stem cells in hostile microenvironments.
Authors: Peng Y, Huang S, Wu Y, Cheng B, Nie X, Liu H, Ma K, Zhou J, Gao D, Feng C, Yang S, Fu X
Stem Cells Dev, 2013-08-30;22(24):3236-51.
Species: Rat
Sample Types: Cell Culture Supernates
-
Diosmin alleviates retinal edema by protecting the blood-retinal barrier and reducing retinal vascular permeability during ischemia/reperfusion injury.
Authors: Tong N, Zhang Z, Zhang W, Qiu Y, Gong Y, Yin L, Qiu Q, Wu X
PLoS ONE, 2013-04-24;8(4):e61794.
Species: Rat
Sample Types: Tissue Homogenates
-
Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity.
Authors: McCloskey M, Wang H, Jiang Y, Smith G, Strange J, Hartnett M
Invest Ophthalmol Vis Sci, 2013-03-21;54(3):2020-6.
Species: Rat
Sample Types: Serum
-
Early cardiac changes in a rat model of prediabetes: brain natriuretic peptide overexpression seems to be the best marker.
Authors: Nunes S, Soares E, Fernandes J, Viana S, Carvalho E, Pereira F, Reis F
Cardiovasc Diabetol, 2013-03-07;12(0):44.
Species: Rat
Sample Types: Serum
-
The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function.
Authors: Takehara, Yuji, Yabuuchi, Akiko, Ezoe, Kenji, Kuroda, Tomoko, Yamadera, Rie, Sano, Chiaki, Murata, Nana, Aida, Takuya, Nakama, Ken, Aono, Fumihito, Aoyama, Naoki, Kato, Keiich, Kato, Osamu
Lab Invest, 2012-11-19;93(2):181-93.
Species: Rat
Sample Types: Cell Culture Supernates
-
Angiopoietins/TIE2 system and VEGF are involved in ovarian function in a DHEA rat model of polycystic ovary syndrome.
Authors: Abramovich D, Irusta G, Bas D, Cataldi NI, Parborell F, Tesone M
Endocrinology, 2012-05-10;153(7):3446-56.
Species: Rat
Sample Types: Tissue Homogenates
-
Angiogenesis is present in experimental autoimmune encephalomyelitis and pro-angiogenic factors are increased in multiple sclerosis lesions.
Authors: Seabrook TJ, Littlewood-Evans A, Brinkmann V, Pollinger B, Schnell C, Hiestand PC
J Neuroinflammation, 2010-12-22;7(0):95.
Species: Rat
Sample Types: Tissue Homogenates
-
Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1.
Authors: Ruiz de Almodovar C, Coulon C, Salin PA, Knevels E, Chounlamountri N, Poesen K, Hermans K, Lambrechts D, Van Geyte K, Dhondt J, Dresselaers T, Renaud J, Aragones J, Zacchigna S, Geudens I, Gall D, Stroobants S, Mutin M, Dassonville K, Storkebaum E, Jordan BF, Eriksson U, Moons L, D'Hooge R, Haigh JJ, Belin MF, Schiffmann S, Van Hecke P, Gallez B, Vinckier S, Chedotal A, Honnorat J, Thomasset N, Carmeliet P, Meissirel C
J. Neurosci., 2010-11-10;30(45):15052-66.
Species: Rat
Sample Types: Tissue Homogenates
-
Overexpressing cellular repressor of E1A-stimulated genes protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis by activation of PI3K/Akt.
Authors: Deng J, Han Y, Yan C, Tian X, Tao J, Kang J, Li S
Apoptosis, 2010-04-01;15(4):463-73.
Species: Rat
Sample Types: Cell Culture Supernates
-
Toll-like receptor 2 mediates mesenchymal stem cell-associated myocardial recovery and VEGF production following acute ischemia-reperfusion injury.
Authors: Abarbanell AM, Wang Y, Herrmann JL
Am. J. Physiol. Heart Circ. Physiol., 2010-02-19;298(5):H1529-36.
Species: Mouse, Rat
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
Poly(Adenosine 5'-diphosphate-ribose) polymerase inhibition counteracts multiple manifestations of experimental type 1 diabetic nephropathy.
Authors: Drel VR, Xu W, Zhang J, Pavlov IA, Shevalye H, Slusher B, Obrosova IG
Endocrinology, 2009-10-23;150(12):5273-83.
Species: Rat
Sample Types: Tissue Homogenates
-
The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model.
Authors: Byfield G, Budd S, Hartnett ME
Invest. Ophthalmol. Vis. Sci., 2009-03-05;50(7):3360-5.
Species: Rat
Sample Types: Tissue Homogenates
-
Phosphomannopentaose sulfate (PI-88) inhibits retinal leukostasis in diabetic rat.
Authors: Ma P, Luo Y, Zhu X, Ma H, Hu J, Tang S
Biochem. Biophys. Res. Commun., 2009-01-23;380(2):402-6.
Species: Rat
Sample Types: Tissue Homogenates
-
The effect of sustained delivery of vascular endothelial growth factor on angiogenesis in tissue-engineered intestine.
Authors: Rocha FG, Sundback CA, Krebs NJ, Leach JK, Mooney DJ, Ashley SW, Vacanti JP, Whang EE
Biomaterials, 2008-04-08;29(19):2884-90.
Species: Rat
Sample Types: Tissue Homogenates
-
Adenosine A(2B) receptor mediates an increase on VEGF-A production in rat kidney glomeruli.
Authors: Valladares D, Quezada C, Montecinos P, Concha II, Yanez AJ, Sobrevia L, San Martin R
Biochem. Biophys. Res. Commun., 2007-12-03;366(1):180-5.
Species: Rat
Sample Types: Cell Culture Supernates
-
Insulin increases retinal hemorrhage in mild oxygen-induced retinopathy in the rat: inhibition by riluzole.
Authors: Yoo MH, Yoon YH, Chung H, Cho KS, Koh JY
Invest. Ophthalmol. Vis. Sci., 2007-12-01;48(12):5671-6.
Species: Rat
Sample Types: Tissue Homogenates
-
Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia.
Authors: Yeh WL, Lin CJ, Fu WM
Mol. Pharmacol., 2007-10-17;73(1):170-7.
Species: Rat
Sample Types: Cell Culture Supernates
-
Triamcinolone acetonide protects the rat retina from STZ-induced acute inflammation and early vascular leakage.
Authors: Kim YH, Choi MY, Kim YS, Park CH, Lee JH, Chung IY, Yoo JM, Choi WS, Cho GJ, Kang SS
Life Sci., 2007-08-31;81(14):1167-73.
Species: Rat
Sample Types: Tissue Homogenates
-
In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power.
Authors: Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR
Surgery, 2007-08-01;142(2):215-21.
Species: Rat
Sample Types: Tissue Homogenates
-
Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression.
Authors: Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM
Mol. Pharmacol., 2007-05-18;72(2):440-9.
Species: Rat
Sample Types: Cell Culture Supernates
-
Bcl-2 engineered MSCs inhibited apoptosis and improved heart function.
Authors: Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G
Stem Cells, 2007-05-03;25(8):2118-27.
Species: Rat
Sample Types: Cell Culture Supernates
-
Improved vascular engraftment and function of autotransplanted pancreatic islets as a result of partial pancreatectomy in the mouse and rat.
Authors: Johansson M, Jansson L, Carlsson PO
Diabetologia, 2007-04-04;50(6):1257-66.
Species: Rat
Sample Types: Plasma
-
Contributory role of VEGF overexpression in endothelin-1-induced cardiomyocyte hypertrophy.
Authors: Shimojo N, Jesmin S, Zaedi S, Otsuki T, Maeda S, Yamaguchi N, Aonuma K, Hattori Y, Miyauchi T
Am. J. Physiol. Heart Circ. Physiol., 2007-03-16;293(1):H474-81.
Species: Rat
Sample Types: Cell Culture Supernates
-
Nicotine-induced vascular endothelial growth factor release via the EGFR-ERK pathway in rat vascular smooth muscle cells.
Authors: Kanda Y, Watanabe Y
Life Sci., 2007-01-17;80(15):1409-14.
Species: Rat
Sample Types: Cell Culture Supernates
-
Combination therapy with the advanced glycation end product cross-link breaker, alagebrium, and angiotensin converting enzyme inhibitors in diabetes: synergy or redundancy?
Authors: Coughlan MT, Thallas-Bonke V, Pete J, Long DM, Gasser A, Tong DC, Arnstein M, Thorpe SR, Cooper ME, Forbes JM
Endocrinology, 2006-11-16;148(2):886-95.
Species: Rat
Sample Types: Urine
-
Specific involvement of SRC family kinase activation in the pathogenesis of retinal neovascularization.
Authors: Werdich XQ, Penn JS
Invest. Ophthalmol. Vis. Sci., 2006-11-01;47(11):5047-56.
Species: Rat
Sample Types: Tissue Homogenates
-
Oxidative stress during peritoneal dialysis: implications in functional and structural changes in the membrane.
Authors: Noh H, Kim JS, Han KH, Lee GT, Song JS, Chung SH, Jeon JS, Ha H, Lee HB
Kidney Int., 2006-06-01;69(11):2022-8.
Species: Rat
Sample Types: Tissue Homogenates
-
Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts.
Authors: Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T
Am. J. Physiol. Heart Circ. Physiol., 2006-04-14;291(3):H1290-8.
Species: Rat
Sample Types: Tissue Homogenates
-
Salvage effect of the vascular endothelial growth factor on chemically induced acute severe liver injury in rats.
Authors: Namisaki T, Yoshiji H, Kojima H, Yoshii J, Ikenaka Y, Noguchi R, Sakurai S, Yanase K, Kitade M, Yamazaki M, Asada K, Uemura M, Nakamura M, Fukui H
J. Hepatol., 2005-09-23;44(3):568-75.
Species: Rat
Sample Types: Tissue Homogenates
-
Antagonism of vascular endothelial growth factor results in microvessel attrition and disorganization of wound tissue.
Authors: Gudehithlu KP, Ahmed N, Wu H, Litbarg NO, Garber SL, Arruda JA, Dunea G, Singh AK
J. Lab. Clin. Med., 2005-04-01;145(4):194-203.
Species: Rat
Sample Types: Granuloma Fluid, Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Rat VEGF Quantikine ELISA Kit
Average Rating: 5 (Based on 4 Reviews)
Have you used Rat VEGF Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Worked very well!
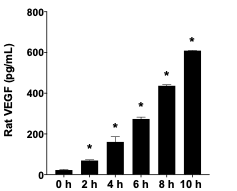
Rat VEGF was evaluated on rat macrophages extracted from blood vessels, after treating the animals with a pro-inflammatory lipid extracted from E. coli. A time-course experiment showed higher VEGF levels after 2-10 h experiment.
We have acquired several times this kit since the results obtained have been reliable. For this experiment, we used this kit to quantify VEGF levels in adult rats (6-8 weeks' age) in small intestine samples (duodenum, jejunum, and ileum).
Quantikine Rat VEGF ELISA kit was used to assess the VEGF levels in rat retinae after Streptozotocin-induced diabetes (Streptozotocin: 60 mg/kg body weight; n=57, male Wistar Rats, 150-180 g. body weight each). The assays were accurate and consistent since the manufacturer guaranteed a low intra-assay difference and sensitivity, which was critical to quantify VEGF in this experiment properly. The figure shows our obtained values of VEGF after treating the rats with several treatments: C (control), DB (Streptozoticin-only), and two P2XtR receptor inhibitors (A740003 and AZ10606120) under control and DB-treatment.