Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF
Now offering a heat stable form of Recombinant Human FGF basic (Catalog # BT-FGFBHS) that retains activity at incubator temperatures and adds flexibility to your media change intervals.
Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF Summary
- R&D Systems E. coli-derived Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein (3718-FB)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Product Specifications
Ala144-Ser288
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
3718-FB
| Formulation | Lyophilized from a 0.2 μm filtered solution in Tris-HCl and NaCl. |
| Reconstitution | Reconstitute at 100-200 μg/mL in sterile PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
Measured in a cell proliferation assay using NR6R‑3T3 mouse fibroblast cells. Raines, E.W.et al. (1985) Methods Enzymol.109:749. The ED50 for this effect is 0.100-0.600 ng/mL.
 View Larger
View Larger
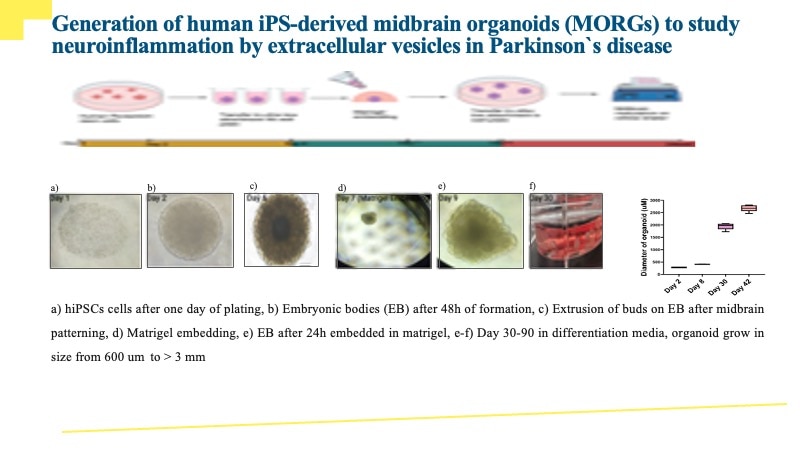
iPSC-derived cerebral organoids (day 45) were cultured using Cultrex UltiMatrix RGF Basement Membrane Extract (BME001-05) and brain organoid culture medium, which includes Recombinant Human FGF-basic (Catalog # 3718-FB) and Recombinant Human Noggin (6057-NG), along with the other reagents listed in the brain organoid culture recipe. Cerebral organoids were stained for Syto6 (blue), Pax6 (red), and Vimentin (green). (A) Image taken at 4x magnification. (B) An enlarged view of the area shown within the white box in part A of the figure. (C) Image taken at 15x magnification. Images courtesy of LifeCanvas Technologies.
 View Larger
View Larger
iPSC-derived cerebral organoids (day 45) were cultured using Cultrex UltiMatrix RGF Basement Membrane Extract (BME001-05) and brain organoid culture medium, which includes Recombinant Human FGF-basic (Catalog # 3718-FG) and Recombinant Human Noggin (6057-NG), along with the other reagents listed in the brain organoid culture recipe. (A) Representative brightfield image of day 30 iPSC-derived cerebral organoids. (B) Cerebral organoids stained for beta III-tubulin (green) and Prox1 (red), and counterstained with DAPI (blue).
Reconstitution Calculator
Background: FGF basic/FGF2/bFGF
FGF basic (also known as FGF-2 and HBGF-2) is an 18-34 kDa, heparin-binding member of the FGF superfamily of molecules (1-3). Superfamily members are characterized by the presence of a centrally placed beta -trefoil structure. FGF acidic (FGF-1) and FGF basic (FGF-2) were the first two identified FGFs, and the designations acidic and basic refer to their relative isoelectric points. Human FGF basic is 288 amino acids (aa) in length. There are multiple start sites, four of which utilize atypical CUG codons, and one that initiates at an AUG start site (4-6). The four CUG start sites generate high molecular weight (HMW) FGF basic. There is a 34 kDa, 288 aa form, a 24 kDa, 210 aa form, a 22.5 kDa, 201 aa form, and a 22 kDa, 196 aa form. All are retained intracellularly, undergo extensive methylation, and possess one or more nuclear localization signals (NLS) (7-9). The AUG initiating form is 18 kDa and 155 aa in length. There is no signal sequence (ss). It is, however, secreted directly through the plasma membrane via a mechanism that appears to be dependent upon tertiary structure (10). In place of a ss, there is purportedly a 9 aa N-terminal prosegment that precedes a 146 aa mature segment (11). Early isolations of 18 kDa bovine FGF basic yielded 146 aa molecules, an effect attributed to the presence of acid proteases (12). The molecule contains a heparin-binding site (aa residues 128-144), and undergoes phosphorylation at Ser117 (13). There is also an ill-defined C-terminal NLS that may be more “functional” (or 3-dimensional) than structural (7). Human 146 aa FGF basic is 97% aa identical to mouse FGF basic (14).
- Sorenson, V. et al. (2006) BioEssays 28:504.
- Kardami, E. et al. (2004) Cardiovasc. Res. 63:458.
- Nugent, M.A. and R.V. Lozzo (2000) Int. J. Biochem. Cell Biol. 32:115.
- Abraham, J.A. et al. (1986) EMBO J. 5:2523.
- Prats, H. et al. (1989) Proc. Natl. Acad. Sci. USA 86:1836.
- Arnaud, E. et al. (1999) Mol. Cell. Biol. 19:505.
- Foletti, A. et al. (2003) Cell. Mol. Life Sci. 60:2254.
- Arese, M. et al. (1999) Mol. Biol. Cell 10:1429.
- Pintucci, G. et al. (1996) Mol. Biol. Cell 7:1249.
- Nickel, W. (2005) Traffic 6:607.
- SwissProt # P09038.
- Klagsbrun, M. et al. (1987) Proc. Natl. Acad. Sci. USA 84:1839.
- Bailly, K. et al. (2000) FASEB J. 14:333.
- Hebert, J.M. et al. (1990) Dev. Biol. 138:454.
Citations for Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
27
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Establishment of human gastrulating stem cells with the capacity of stable differentiation into multiple gastrulating cell types
Authors: Huang, M;Chen, M;Yuan, G;Cui, Y;Shen, B;Liu, Z;Zhang, B;Chen, J;Chen, D;Qiu, S;Zhang, Y;Liu, L;Qin, L;Zhu, Y;Liu, J;Zhang, H;Wu, J;Yuan, Y;Sha, J;
Cell research
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
BFGF mitigates CTX-induced ovarian cytotoxicity via SERPINE1/HIF-1 and Nrf-2/HO-1 signaling pathways
Authors: Li, Y;Zhang, L;Lv, H;Mei, C;Hu, M;
Journal of ovarian research
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A Transcriptomic Comparison of the HD10.6 Human Sensory Neuron-Derived Cell Line with Primary and iPSC Sensory Neurons
Authors: Al-Abbasi, Z;Bhuiyan, SA;Renthal, W;Molliver, DC;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Reconstructing signaling histories of single cells via perturbation screens and transfer learning
Authors: Hutchins, NT;Meziane, M;Lu, C;Mitalipova, M;Fischer, D;Li, P;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
c-Rel drives pancreatic cancer metastasis through Fibronectin-Integrin signaling-induced isolation stress resistance and EMT activation
Authors: Bak?rdö?en, D;Görgülü, K;Xin, J;Alcalá, S;Ruiz-Cañas, L;Frank, K;Wu, N;Diakopoulos, KN;Dai, C;Öztürk, H;Demircio?lu, D;Peschke, K;Ranjan, R;Fusco, F;Martinez-Useros, J;Fernandez-Aceñero, MJ;Chhabra, NF;López-Gil, JC;Ai, J;Ruess, DA;Kaya-Aksoy, E;Steiger, K;Schmidt, F;Kohlmann, L;Berninger, A;Schmid, RM;Reichert, M;Adli, M;Lesina, M;Sainz, B;Algül, H;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Spheroid
Applications: Bioassay -
Isolation, culture, and characterization of primary endothelial cells and pericytes from mouse sciatic nerve
Authors: Huang, Y;Jin, HR;Liu, FY;Fridayana, FR;Vo, MN;Ryu, JK;Yin, GN;
Journal of neuroscience methods
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
LM11a-31 Inhibits p75 Neurotrophin Receptor (p75 NTR ) Cleavage and is Neuroprotective in a Cell Culture Model of Parkinson's Disease
Authors: Pokharel, PV;Newchurch, AM;Overby, SC;Spease, CA;Darzi, LG;Kraemer, BR;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
linc-ADAIN, a human adipose lincRNA, regulates adipogenesis by modulating KLF5 and IL-8 mRNA stability
Authors: O'Reilly, ME;Ho, S;Coronel, J;Zhu, L;Liu, W;Xue, C;Kim, E;Cynn, E;Matias, CV;Soni, RK;Wang, C;Ionita-Laza, I;Bauer, RC;Ross, L;Zhang, Y;Corvera, S;Fried, SK;Reilly, MP;
Cell reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
METTL16 promotes liver cancer stem cell self-renewal via controlling ribosome biogenesis and mRNA translation
Authors: Xue, M;Dong, L;Zhang, H;Li, Y;Qiu, K;Zhao, Z;Gao, M;Han, L;Chan, AKN;Li, W;Leung, K;Wang, K;Pokharel, SP;Qing, Y;Liu, W;Wang, X;Ren, L;Bi, H;Yang, L;Shen, C;Chen, Z;Melstrom, L;Li, H;Timchenko, N;Deng, X;Huang, W;Rosen, ST;Tian, J;Xu, L;Diao, J;Chen, CW;Chen, J;Shen, B;Chen, H;Su, R;
Journal of hematology & oncology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
CRISPR screening identifies mechanisms of resistance to KRASG12C and SHP2 inhibitor combinations in non-small cell lung cancer
Authors: Prahallad, A;Weiss, A;Voshol, H;Kerr, G;Sprouffske, K;Yuan, T;Ruddy, D;Meistertzheim, M;Kazic-Legueux, M;Kottarathil, T;Piquet, M;Cao, Y;Martinuzzi-Duboc, L;Buhles, A;Alder, F;Mannino, S;Tordella, L;Sansregret, L;Maira, SM;Graus Porta, D;Fedele, C;Brachmann, SM;
Cancer research
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Short C-terminal Musashi-1 proteins regulate pluripotency states in embryonic stem cells
Authors: Chen, Y;Chen, Y;Li, Q;Liu, H;Han, J;Zhang, H;Cheng, L;Lin, G;
Cell reports
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
DISTINCT METABOLIC STATES DIRECT RETINAL PIGMENT EPITHELIUM CELL FATE DECISIONS
Authors: Perez-Estrada, JR;Tangeman, JA;Proto-Newton, M;Sanaka, H;Smucker, B;Del Rio-Tsonis, K;
bioRxiv : the preprint server for biology
Species: Chicken
Sample Types: Whole Tissue
Applications: Bioassay -
TGF?1-RCN3-TGFBR1 loop facilitates pulmonary fibrosis by orchestrating fibroblast activation
Authors: Wu, M;Wang, Z;Shi, X;Zan, D;Chen, H;Yang, S;Ding, F;Yang, L;Tan, P;Ma, RZ;Wang, J;Ma, L;Ma, Y;Jin, J;
Respiratory research
Species: Human
Sample Types: Transfected Whole Cells
Applications: Bioassay -
PI16+ reticular cells in human palatine tonsils govern T cell activity in distinct subepithelial niches
Authors: De Martin, A;Stanossek, Y;L�tge, M;Cadosch, N;Onder, L;Cheng, HW;Brandstadter, JD;Maillard, I;Stoeckli, SJ;Pikor, NB;Ludewig, B;
Nature immunology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
CD44 promotes angiogenesis in myocardial infarction through regulating plasma exosome uptake and further enhancing FGFR2 signaling transduction.
Authors: Zhang Q, Chen L, Huang L, Cheng H, Wang L, Xu L, Hu D, He C, Fu C, Wei Q
Mol Med, 2022-12-03;28(1):145.
Species: Human
Sample Types: Transfected Whole Cells
Applications: Bioassay -
A common transcriptional mechanism involving R-loop and RNA abasic site regulates an enhancer RNA of APOE
Authors: JA Watts, C Grunseich, Y Rodriguez, Y Liu, D Li, JT Burdick, A Bruzel, RJ Crouch, RW Mahley, SH Wilson, VG Cheung
Nucleic Acids Research, 2022-11-28;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
iPSCs derived from esophageal atresia patients reveal SOX2 dysregulation at the anterior foregut stage
Authors: S Raad, A David, M Sagniez, B Paré, Z Orfi, NA Dumont, MA Smith, C Faure
Disease Models & Mechanisms, 2022-11-28;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A Stage-Specific OTX2 Regulatory Network and Maturation-Associated Gene Programs Are Inherent Barriers to RPE Neural Competency.
Authors: Tangeman J, Perez-Estrada J, Van Zeeland E, Liu L, Danciutiu A, Grajales-Esquivel E, Smucker B, Liang C, Del Rio-Tsonis K
Front Cell Dev Biol, 2022-04-19;10(0):875155.
Species: Chicken
Sample Types: Whole Tissue
Applications: Bioassay -
Physical and functional interactome atlas of human receptor tyrosine kinases.
Authors: Salokas K, Liu X, Ohman T, Chowdhury I, Gawriyski L, Keskitalo S, Varjosalo M
EMBO Rep, 2022-04-05;23(6):e54041.
Species: Human
Sample Types: Transfected Whole Cells
Applications: Bioassay -
The Role of Interstitial Fluid Pressure in Cerebral Porous Biomaterial Integration
Authors: F Bonini, S Mosser, FM Mor, A Boutabla, P Burch, A Béduer, A Roux, T Braschler
Brain sciences, 2022-03-22;12(4):.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Coactivation of GSK3beta and IGF-1 Attenuates Amyotrophic Lateral Sclerosis Nerve Fiber Cytopathies in SOD1 Mutant Patient-Derived Motor Neurons
Authors: HC Ting, HI Yang, HJ Harn, IM Chiu, HL Su, X Li, MF Chen, TJ Ho, CA Liu, YJ Tsai, TW Chiou, SZ Lin, CY Chang
Cells, 2021-10-16;10(10):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Bioassay -
The Use of Human Serum Samples to Study Malignant Transformation: A Pilot Study
Authors: AN Holowatyj, B Gigic, CA Warby, J Ose, T Lin, P Schrotz-Ki, CM Ulrich, JJ Bernard
Cells, 2021-10-06;10(10):.
Species: Human
Sample Types: Serum
Applications: Bioassay -
Regulation of prefrontal patterning and connectivity by retinoic acid
Authors: M Shibata, K Pattabiram, B Lorente-Ga, D Andrijevic, SK Kim, N Kaur, SK Muchnik, X Xing, G Santpere, AMM Sousa, N Sestan
Nature, 2021-10-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Cell Culture -
Glioblastoma patient-derived cell-based phenotypic drug screening and identification of possible action mechanisms through proteomic analysis.
Authors: Kim Y, Kim H, Jung D, Kang D, Nam D, Nam H, Cho H
STAR Protoc, 2021-09-24;2(4):100849.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A novel antibody for the detection of alternatively spliced secreted KLOTHO isoform in human plasma
Authors: S Jadhav, S Tripathi, A Chandrekar, SS Waikar, LL Hsiao
PLoS ONE, 2021-01-22;16(1):e0245614.
Species: Human
Sample Types: Protein
Applications: ELISA Capture -
Low dose amiodarone reduces tumor growth and angiogenesis
Authors: E Steinberg, A Fluksman, C Zemmour, K Tischenko, A Karsch-Blu, Y Brill-Karn, AE Birsner, RJ D'Amato, O Benny
Sci Rep, 2020-10-22;10(1):18034.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Generating Patient-Derived Gliomas within Cerebral Organoids.
Authors: Linkous A, Fine H
STAR Protoc, 2020-06-03;1(1):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
-
What receptors does FGF basic bind?
FGF receptor specificity has been reviewed in multiple citations. Please find more information at: //www.rndsystems.com/resources/articles/fibroblast-growth-factors-and-their-receptors
-
Does human FGF basic show activity on mouse cells?
Yes, it does. The bioassay uses NR-6 mouse fibroblast cells. There is 95% homology between the human and mouse protein and 98% homology between the human and mouse receptor.
Reviews for Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Recombinant Human FGF basic/FGF2/bFGF (145 aa) Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: it works fine