Recombinant Human FGF basic/FGF2/bFGF Protein, Animal-Free
Recombinant Human FGF basic/FGF2/bFGF Protein, Animal-Free Summary
Product Specifications
Ala135-Ser288
Produced using non-animal reagents in an animal-free laboratory.
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
BT-FGFB-AFL
| Formulation | Lyophilized from a 0.2 μm filtered solution in HEPES and Sodium Sulfate with Trehalose. |
| Reconstitution | Reconstitute 25 μg size at 250 μg/mL and others at 500 μg/mL in sterile water. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
The lot-to-lot consistency of Animal-Free™ Recombinant Human FGF basic/FGF2/bFGF Protein (Catalog # BT-FGFB-AFL) was assessed by testing the ability of two independent lots of the protein to stimulate proliferation of the NR6R-3T3 mouse fibroblast cell line. The ED50 for this effect is 0.100-1.00 ng/mL.
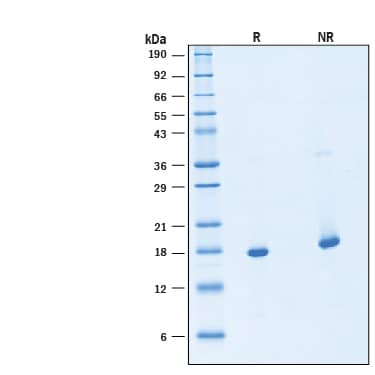 View Larger
View Larger
2 μg/lane of Animal-Free™ Recombinant Human FGF basic/FGF2/bFGF Protein (Catalog # BT-FGFB-AFL) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 18 kDa.
Reconstitution Calculator
Background: FGF basic/FGF2/bFGF
FGF basic (also known as FGF-2 and HBGF-2) is an 18-34 kDa, heparin-binding member of the FGF superfamily of molecules (1-3). Superfamily members are characterized by the presence of a centrally placed beta -trefoil structure. FGF acidic (FGF-1) and FGF basic (FGF-2) were the first two identified FGFs, and the designations acidic and basic refer to their relative isoelectric points. Human FGF basic is 288 amino acids (aa) in length. There are multiple start sites, four of which utilize atypical CUG codons, and one that initiates at an AUG start site (4-6). The four CUG start sites generate high molecular weight (HMW) FGF basic. There is a 34 kDa, 288 aa form, a 24 kDa, 210 aa form, a 22.5 kDa, 201 aa form, and a 22 kDa, 196 aa form. All are retained intracellularly, undergo extensive methylation, and possess one or more nuclear localization signals (NLS) (7-9). The AUG initiating form is 18 kDa and 155 aa in length. There is no signal sequence (ss). It is, however, secreted directly through the plasma membrane via a mechanism that appears to be dependent upon tertiary structure (10). In place of a ss, there is purportedly a 9 aa N-terminal prosegment that precedes a 146 aa mature segment (11). Early isolations of 18 kDa bovine FGF basic yielded 146 aa molecules, an effect attributed to the presence of acid proteases (12). The molecule contains a heparin-binding site (aa residues 128-144), and undergoes phosphorylation at Ser117 (13). There is also an ill-defined C-terminal NLS that may be more “functional” (or 3-dimensional) than structural (7). Human 146 aa FGF basic is 97% aa identical to mouse FGF basic (14).
- Sorenson, V. et al. (2006) BioEssays 28:504.
- Kardami, E. et al. (2004) Cardiovasc. Res. 63:458.
- Nugent, M.A. and R.V. Lozzo (2000) Int. J. Biochem. Cell Biol. 32:115.
- Abraham, J.A. et al. (1986) EMBO J. 5:2523.
- Prats, H. et al. (1989) Proc. Natl. Acad. Sci. USA 86:1836.
- Arnaud, E. et al. (1999) Mol. Cell. Biol. 19:505.
- Foletti, A. et al. (2003) Cell. Mol. Life Sci. 60:2254.
- Arese, M. et al. (1999) Mol. Biol. Cell 10:1429.
- Pintucci, G. et al. (1996) Mol. Biol. Cell 7:1249.
- Nickel, W. (2005) Traffic 6:607.
- SwissProt # P09038.
- Klagsbrun, M. et al. (1987) Proc. Natl. Acad. Sci. USA 84:1839.
- Bailly, K. et al. (2000) FASEB J. 14:333.
- Hebert, J.M. et al. (1990) Dev. Biol. 138:454.
Manufacturing Specifications
Animal-Free Manufacturing ConditionsOur dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
Purification
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers in a dedicated cold storage room.
- Low Endotoxin Level.
- No impairment of biological activity.
- High quality product obtained under stringent conditions.
- For ex vivo research or bioproduction, additional documentation can be provided.
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human FGF basic/FGF2/bFGF Protein, Animal-Free
There are currently no reviews for this product. Be the first to review Recombinant Human FGF basic/FGF2/bFGF Protein, Animal-Free and earn rewards!
Have you used Recombinant Human FGF basic/FGF2/bFGF Protein, Animal-Free?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image



