Recombinant Human IL-2 GMP Protein, CF
New! Bypass reconstitution steps by using a liquid formulation of GMP-grade Recombinant Human IL-2. Find out more here.
Recombinant Human IL-2 GMP Protein, CF Summary
- IL-2 Manufactured in Bio-Techne's new GMP facility
- Lot-to-lot consistency
- Stringent guidelines for patient safety
- Scalability necessary to support successful therapeutics
- Learn more about manufacturing in our new GMP facility
- Test it in your process! Request a sample of GMP IL-2
Product Specifications
The ED50 for this effect is 0.0300-0.250 ng/mL.
The specific activity of recombinant human IL-2 is >5.00 x 106 IU/mg, which is calibrated against an internal reference standard value assigned against the human IL-2 WHO International Standard (NIBSC code: 86/500).
Ala21 - Thr153 (Cys145Ser), with and without an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.
Analysis
Product Datasheets
BT-002-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in Sodium Acetate with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in sterile deionized water. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
BT-002-GMP/LQ
| Formulation | Supplied as a 0.2 μm filtered solution in Sodium Acetate. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
GMP-grade Recombinant Human IL-2 (Catalog # BT-002-GMP) as measured in a cell proliferation assay using CTLL-2 mouse cytotoxic T cells. The ED50 for this effect is 0.0300-0.250 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of GMP IL-2.
 View Larger
View Larger
Equivalent bioactivity of GMP (Catalog # BT-002-GMP) and Animal-Free (BT-002-AFL) grades of Recombinant Human IL-2 as measured in a cell proliferation assay using CTLL-2 mouse cytotoxic T cells (orange and green, respectively).
 View Larger
View Larger
2 μg/lane of Recombinant Human IL-2 GMP Protein (Catalog # BT-002-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 13 kDa.
 View Larger
View Larger
Two samples of GMP-grade human IL-2 (Catalog number BT-002-GMP, green circles) were interpolated with the human IL-2 Quantikine ELISA (D2050) standard curve (black circles) using 4PL logistic regression analysis. The human IL-2 Quantikine ELISA has an assay range of 31.2-2,000 pg/mL.
 View Larger
View Larger
Thirteen samples of GMP-grade human IL-2 (Catalog number BT-002-GMP, green circles) were interpolated with the Simple Plex Human IL-2 cartridge (SPCKB-PS-000295) standard curve (black circles) using 4PL logistic regression analysis. The Simple Plex IL-2 cartridge has an assay range of 0.54-2,050 pg/mL.
 View Larger
View Larger
Three independent lots of Recombinant Human IL-2 GMP Protein (Catalog # BT-002-GMP) were analyzed by Maurice CE-SDS PLUS (IS is an Internal Standard). A gel-like representation of the purity analysis data (inset) can be obtained from the Lane View feature in Compass software for iCE. Profiles from the three runs were superimposed, showing excellent manufacturing consistency.
 View Larger
View Larger
Three independent lots of Recombinant Human IL-2 GMP Protein (Catalog # BT-002-GMP) were analyzed by Maurice icIEF using native fluorescence detection (Mkr 5.85 and 9.99 are pI Markers). Profiles from the three runs were superimposed, showing excellent manufacturing consistency.
 View Larger
View Larger
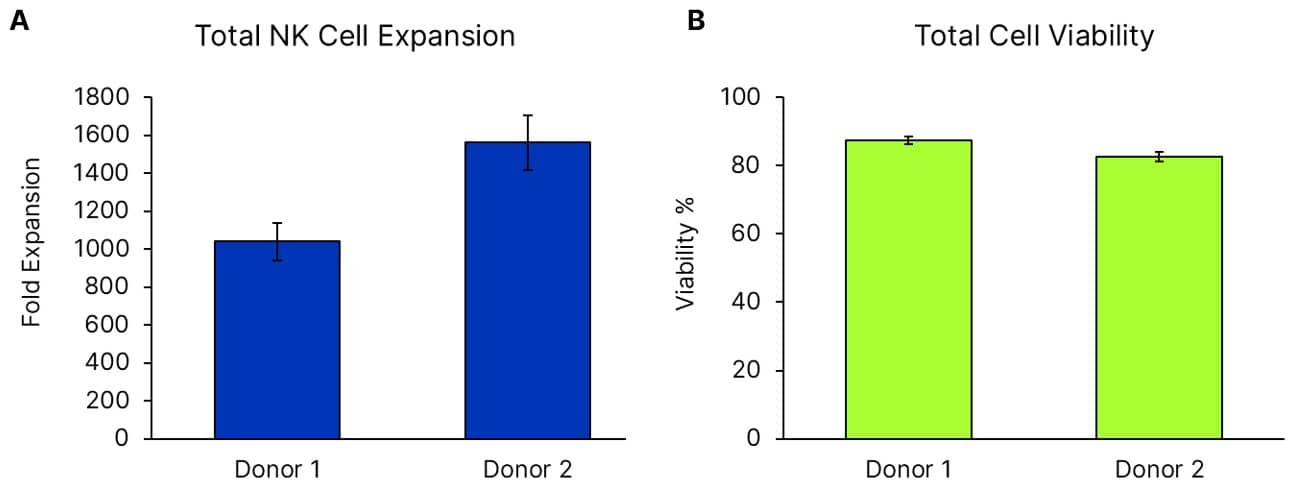
Donor PBMCs were grown using ExCellerate NK Cell Expansion Media (Catalog # CCM037) supplemented with IL-2 (Catalog # BT-002-GMP), IL-12 (Catalog # 10018-IL), IL-15 (Catalog # BT-015-GMP), IL-18 (Catalog # BT-018-GMP), and IL-21 (Catalog # BT-021-GMP) on plates coated with anti-human NKp46 (Catalog # MAB1850). PBMCs were plated to contain 1.8e4 NK cells as determined by flow cytometry on day 0. On day 5, 0.1e6 total cells were taken to seed a 6 well plate. Viability, expansion, and NK purity were analyzed on day 9, with n=5 +/- SEM. A: Total expansion of CD56+ CD3- cells between day 0 and day 9. B: PBMCs grown in ExCellerate NK Cell Expansion Media supplemented with cytokines exhibit viability greater than 80% at day 9.
 View Larger
View Larger
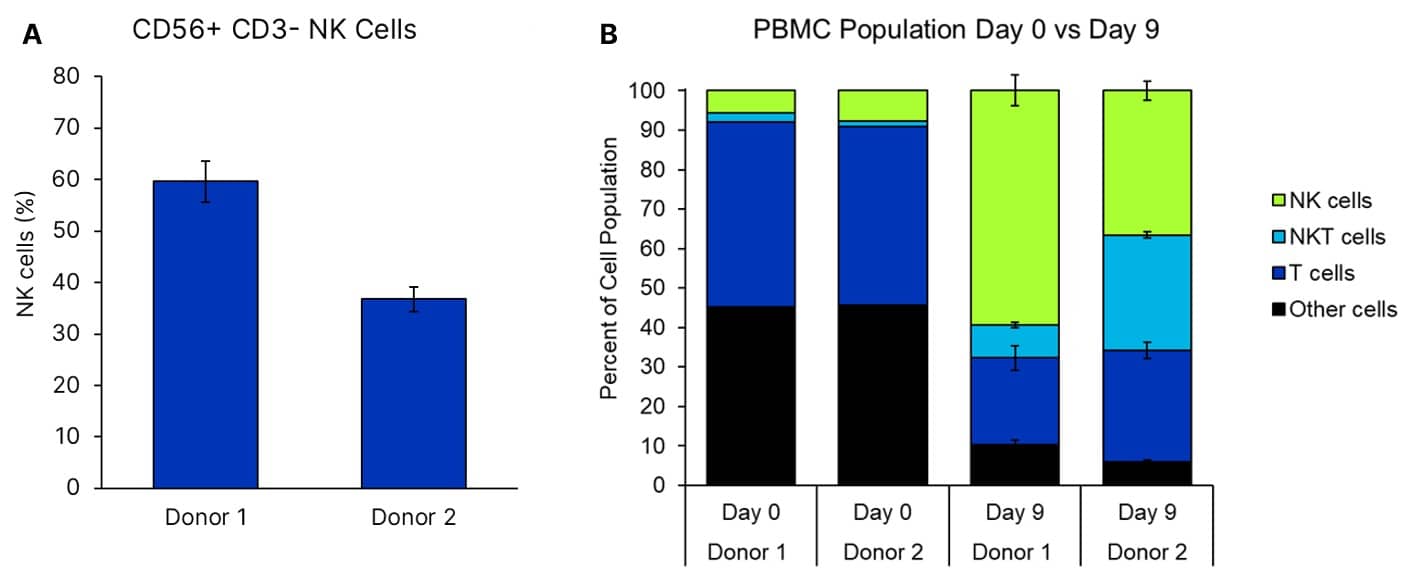
Donor PBMCs were grown using ExCellerate NK Cell Expansion Media (Catalog # CCM037) supplemented with IL-2 (Catalog # BT-002-GMP), IL-12 (Catalog # 10018-IL), IL-15 (Catalog # BT-015-GMP), IL-18 (Catalog # BT-018-GMP), and IL-21 (Catalog # BT-021-GMP) on plates coated with anti-human NKp46 (Catalog # MAB1850). PBMCs were plated to contain 1.8e4 NK cells as determined by flow cytometry on day 0. On day 5, 0.1e6 total cells were taken to seed a 6 well plate. Viability, expansion, and NK purity were analyzed on day 9, with n=5 +/- SEM for day 9; n=1 for day 0. A: Purity of NK cells at day 9. B: NK ExCellerate media preferentially expands CD56+ NK cells. Percentage of CD56+ cells in culture rose from less than 10% in both donors to more than 60% by day 9.
Reconstitution Calculator
Background: IL-2
Recombinant Interleukin-2 (IL-2) is expressed in E. coli and has been engineered to contain the serine for cysteine substitution found in Proleukin® (aldesleukin). Recombinant IL-2 is widely used in cell culture for the expansion of T cells.
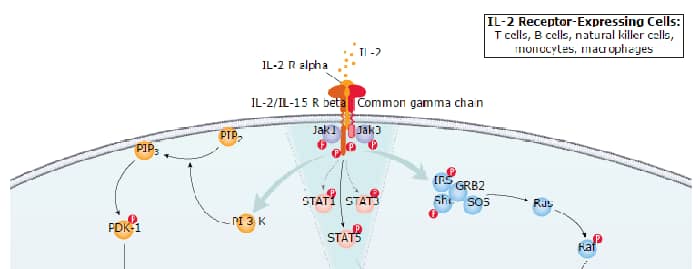
IL-2 is expressed by CD4+ and CD8+ T cells, gamma δ T cells, B cells, dendritic cells, and eosinophils (1 - 3). Mature human IL-2 shares 56% and 66% amino acid (aa) sequence identity with mouse and rat IL-2, respectively. Human and mouse IL-2 exhibit cross-species activity (4). The receptor for IL-2 consists of three subunits that are present on the cell surface in varying preformed complexes (5 - 7). The 55 kDa IL-2 R alpha is specific for IL-2 and binds with low affinity. The 75 kDa IL-2 R beta, which is also a component of the IL-15 receptor, binds IL-2 with intermediate affinity. The 64 kDa common gamma chain gamma c/IL-2 R gamma, which is shared with the receptors for IL-4, -7, -9, -15, and -21, does not independently interact with IL-2. Upon ligand binding, signal transduction is performed by both IL-2 R beta and gamma c.
IL-2 is best known for its autocrine and paracrine activity on T cells. It drives resting T cells to proliferate and induces IL-2 and IL-2 R alpha synthesis (1, 2). It contributes to T cell homeostasis by promoting the Fas-induced death of naïve CD4+ T cells but not activated CD4+ memory lymphocytes (8). IL-2 plays a central role in the expansion and maintenance of regulatory T cells, although it inhibits the development of Th17 polarized cells (9 - 11). Thus, IL-2 may be a key cytokine in the natural suppression of autoimmunity (12, 13).
IL-2 expression and concentration can have either immunostimulatory effects at high doses or immunosuppressive effects at low doses due to its preferential binding to different receptor subunits expressed by various immune cell types. This has led to the generation of recombinant IL-2 variants aimed at modifying IL-2 receptor binding for increased antitumor efficacy (14, 15). These variants are typically used in combination with immune checkpoint inhibitors instead of as a monotherapy (14). IL-2 can be genetically engineered to express in NK cells for CAR T cell therapies, and in combination with other cytokines like IL-15, can increase cell viability and proliferation (16). In addition to adoptive cell transfer and checkpoint blockade inhibitors, cancer vaccines that boost immune responses have been combined with IL-2 treatment with promising results in recent studies (15).- Ma, A. et al. (2006) Annu. Rev. Immunol. 24:657.
- Gaffen, S.L. and K.D. Liu (2004) Cytokine 28:109.
- Taniguchi, T. et al. (1983) Nature 302:305.
- Mosmann, T.R. et al. (1987) J. Immunol. 138:1813.
- Liparoto, S.F. et al. (2002) Biochemistry 41:2543.
- Wang, X. et al. (2005) Science 310:1159.
- Bodnar, A. et al. (2008) Immunol. Lett. 116:117.
- Jaleco, S. et al. (2003) J. Immunol. 171:61.
- Malek, T.R. (2003) J. Leukoc. Biol. 74:961.
- Laurence, A. et al. (2007) Immunity 26:371.
- Kryczek, I. et al. (2007) J. Immunol. 178:6730.
- Afzali, B. et al. (2007) Clin. Exp. Immunol. 148:32.
- Fehervari, Z. et al. (2006) Trends Immunol. 27:109.
- Xue, D. et al. (2021) Antib Ther. 4:123.
- Wolfarth, A.A. et al. (2022) Immune Netw. 22:e5.
- Koehl, U. et al. (2015) Oncoimmunology. 5:e1115178.
- Marsman, C. et al. (2022) Front. Immunol. 13:815449.
- Chamucero-Millares, J.A.et al. (2021) Cell Immunol. 360:104257.
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices
Proleukin is a registered trademark of Clinigen Holdings Limited. Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.Citation for Recombinant Human IL-2 GMP Protein, CF
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
1 Citation: Showing 1 - 1
-
Deciphering regulation of FOXP3 expression in human conventional T cells
Authors: Umhoefer, JM;Arce, MM;Dajani, R;Belk, JA;Mowery, CT;Nguyen, V;Gowen, BG;Simeonov, DR;Curie, GL;Corn, JE;Chang, HY;Marson, A;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human IL-2 GMP Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Human IL-2 GMP Protein, CF and earn rewards!
Have you used Recombinant Human IL-2 GMP Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image