Recombinant Human MMP-2 Protein, CF Summary
- R&D Systems CHO-derived Recombinant Human MMP-2 Protein (902-MP)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Product Specifications
Ile34-Cys660
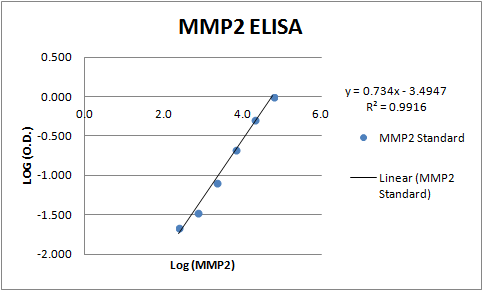
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
902-MP
| Formulation | Supplied as a 0.2 μm filtered solution in Tris, CaCl2, NaCl and Brij-35. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Assay Procedure
- Assay Buffer: 50 mM Tris, 10 mM CaCl2, 150 mM NaCl, 0.05% (w/v) Brij 35, pH 7.5 (TCNB)
- Recombinant Human MMP-2 (rhMMP-2) (Catalog # 902-MP)
- p-aminophenylmercuric acetate (APMA), (Sigma, Catalog # A-9563), 100 mM stock in DMSO
- Fluorogenic Peptide Substrate I: MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2 (Catalog # ES001)
- F16 Black Maxisorp Plate (Nunc, Catalog # 475515)
- Fluorescent Plate Reader (Model: SpectraMax Gemini EM by Molecular Devices) or equivalent
- Dilute rhMMP-2 to 100 µg/mL in Assay Buffer.
- Activate rhMMP-2 by adding APMA to a final concentration of 1 mM.
- Incubate at 37 °C for 1 hour.
- Dilute activated rhMMP-2 to 0.2 ng/µL in Assay Buffer.
- Dilute Substrate to 20 µM in Assay Buffer.
- Load into a black well plate 50 µL of the 0.2 ng/µL rhMMP-2 and start the reaction by adding 50 µL of 20 µM Substrate. Include a Substrate Blank containing 50 µL Assay Buffer and 50 µL of 20 µM Substrate.
- Read at excitation and emission wavelengths of 320 nm and 405 nm, respectively, in kinetic mode for 5 minutes.
- Calculate specific activity:
|
Specific Activity (pmol/min/µg) = |
Adjusted Vmax* (RFU/min) x Conversion Factor** (pmol/RFU) |
| amount of enzyme (µg) |
*Adjusted for Substrate Blank
**Derived using calibration standard MCA-Pro-Leu-OH (Bachem, Catalog # M-1975).
Per Well:- rhMMP-2: 0.010 µg
- Substrate: 10 µM
Reconstitution Calculator
Background: MMP-2
Matrix metalloproteinases are a family of zinc and calcium dependent endopeptidases with the combined ability to degrade all the components of the extracellular matrix. MMP-2 (gelatinase A), a type IV collagenase, can degrade a broad range of substrates including type IV, V, VII and X collagens as well as elastin and fibronectin. It is believed to act synergistically with interstitial collagenase (MMP-1) in the degradation of fibrillar collagens as it degrades their denatured gelatin forms. MMP-2 has been shown to be associated with many connective tissue cells as well as neutrophils, macrophages and monocytes. Structurally, MMP-2 may be divided into several distinct domains: a pro-domain which is cleaved upon activation; a catalytic domain containing the zinc binding site; a fibronectin-like domain thought to play a role in substrate targeting; and a carboxyl terminal (hemopexin-like) domain containing 2 N-linked glycosylation sites.
Citations for Recombinant Human MMP-2 Protein, CF
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
29
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
An Investigation of the Altered Textural Property in Woody Breast Myopathy Using an Integrative Omics Approach
Authors: AA Welter, WJ Wu, R Maurer, TG O'Quinn, MD Chao, DL Boyle, ER Geisbrecht, SD Hartson, BC Bowker, H Zhuang
Frontiers in Physiology, 2022-03-17;13(0):860868.
Species: Human
Sample Types: Recombinant Protein
Applications: Bioassay -
Protease-Triggered Release of Stabilized CXCL12 from Coated Scaffolds in an Ex Vivo Wound Model
Authors: S Spiller, T Wippold, K Bellmann-S, S Franz, A Saalbach, U Anderegg, AG Beck-Sicki
Pharmaceutics, 2021-10-01;13(10):.
Species: Human
Sample Types: Protein
Applications: Enzyme Assay -
Novel Ex Vivo Zymography Approach for Assessment of Protease Activity in Tissues with Activatable Antibodies
Authors: B Howng, MB Winter, C LePage, I Popova, M Krimm, O Vasiljeva
Pharmaceutics, 2021-09-02;13(9):.
Species: Human
Sample Types: Peptides
Applications: Bioassay -
Mapping specificity, cleavage entropy, allosteric changes and substrates of blood proteases in a high-throughput screen
Authors: F Uliana, M Vizovišek, L Acquasalie, R Ciuffa, A Fossati, F Frommelt, S Goetze, B Wollscheid, M Gstaiger, V De Filippi, U Auf dem Ke, R Aebersold
Nature Communications, 2021-03-16;12(1):1693.
Species: Human
Sample Types: Cell Lysates
Applications: Bioassay -
Matrix metalloproteinase (MMP)-2, MMP-9, semen quality and sperm longevity in fractionated stallion semen
Authors: M Kareskoski, J Vakkamäki, K Laukkanen, M Palviainen, A Johannisso, T Katila
Theriogenology, 2021-02-02;164(0):93-99.
Species: Equine
Sample Types: Semen
Applications: Gelatin Zymography Control -
Kallikrein-Related Peptidase 14 Activates Zymogens of Membrane Type Matrix Metalloproteinases (MT-MMPs)-A CleavEx Based Analysis
Authors: K Falkowski, E Bielecka, IB Thøgersen, O Boche?ska, K P?aza, M Kali?ska, L S?siadek, M Magoch, A P?cak, M Wi?niewska, N Gruba, M Wysocka, A Wojtysiak, M Brzezi?ska, K Sychowska, A Pejkovska, M Rehders, G Butler, CM Overall, K Brix, G Dubin, A Lesner, A Kozik, JJ Enghild, J Potempa, T Kantyka
Int J Mol Sci, 2020-06-19;21(12):.
Species: Human
Sample Types: Recombinant Protein
Applications: Bioassay -
Biodistribution of Nanostructured Lipid Carriers in Mice Atherosclerotic Model
Authors: L Devel, G Almer, C Cabella, F Beau, M Bernes, P Oliva, F Navarro, R Prassl, H Mangge, I Texier
Molecules, 2019-09-26;24(19):.
Species: Human
Sample Types: Peptide
Applications: Bioassay -
Eradication of unresectable liver metastasis through induction of tumour specific energy depletion
Authors: D Huo, J Zhu, G Chen, Q Chen, C Zhang, X Luo, W Jiang, X Jiang, Z Gu, Y Hu
Nat Commun, 2019-07-11;10(1):3051.
Species: Human
Sample Types: Nanoparticles
Applications: Bioassay -
Glycosaminoglycans influence enzyme activity of MMP2 and MMP2/TIMP3 complex formation - Insights at cellular and molecular level
Authors: G Ruiz-Gómez, S Vogel, S Möller, MT Pisabarro, U Hempel
Sci Rep, 2019-03-20;9(1):4905.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Enzyme Assay -
Reduced vasorin enhances angiotensin II signaling within the aging arterial wall
Authors: G Pintus, R Giordo, Y Wang, W Zhu, SH Kim, L Zhang, L Ni, J Zhang, R Telljohann, KR McGraw, RE Monticone, C Ferris, L Liu, M Wang, EG Lakatta
Oncotarget, 2018-06-05;9(43):27117-27132.
Species: Rat
Sample Types: Protein
Applications: Enzyme Assay -
The enzymatic processing of ?-dystroglycan by MMP-2 is controlled by two anchoring sites distinct from the active site
Authors: M Gioia, GF Fasciglion, D Sbardella, F Sciandra, M Casella, S Camerini, M Crescenzi, A Gori, U Tarantino, P Cozza, A Brancaccio, M Coletta, M Bozzi
PLoS ONE, 2018-02-15;13(2):e0192651.
Applications: Bioassay -
Downregulation of monocytic differentiation via modulation of CD147 by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors
Authors: MV Sasidhar, SK Chevooru, O Eickelberg, HP Hartung, O Neuhaus
PLoS ONE, 2017-12-18;12(12):e0189701.
Species: Human
Sample Types: Recombinant Protein
Applications: Zymography -
Gelatinases A and B and Antioxidant Enzyme Activity in the Early Phase of Acute Myocardial Infarction
Authors: K Gopcevic, B Rovcanin, D Kekic, D Milasinovi, G Kocic, I Stojanovic
Folia Biol. (Praha), 2017-01-01;63(1):20-26.
Applications: Zymography -
Plantamajoside, a potential anti-tumor herbal medicine inhibits breast cancer growth and pulmonary metastasis by decreasing the activity of matrix metalloproteinase-9 and -2.
Authors: Pei S, Yang X, Wang H, Zhang H, Zhou B, Zhang D, Lin D
BMC Cancer, 2015-12-16;15(0):965.
Species: Human
Sample Types: Recombinant Protein
Applications: Bioassay -
Stanniocalcin-1 Potently Inhibits the Proteolytic Activity of the Metalloproteinase Pregnancy-associated Plasma Protein-A.
Authors: Kloverpris S, Mikkelsen J, Pedersen J, Jepsen M, Laursen L, Petersen S, Oxvig C
J Biol Chem, 2015-07-20;290(36):21915-24.
Species: Human
Sample Types: Protein
Applications: Enzyme Assay -
Protease-degradable electrospun fibrous hydrogels.
Authors: Wade R, Bassin E, Rodell C, Burdick J
Nat Commun, 2015-03-23;6(0):6639.
Applications: Bioassay -
ADAM8 as a drug target in pancreatic cancer.
Authors: Schlomann U, Koller G, Conrad C, Ferdous T, Golfi P, Garcia A, Hofling S, Parsons M, Costa P, Soper R, Bossard M, Hagemann T, Roshani R, Sewald N, Ketchem R, Moss M, Rasmussen F, Miller M, Lauffenburger D, Tuveson D, Nimsky C, Bartsch J
Nat Commun, 2015-01-28;6(0):6175.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Investigation of a MMP-2 activity-dependent anchoring probe for nuclear imaging of cancer.
Authors: Temma, Takashi, Hanaoka, Hirofumi, Yonezawa, Aki, Kondo, Naoya, Sano, Kohei, Sakamoto, Takeharu, Seiki, Motoharu, Ono, Masahiro, Saji, Hideo
PLoS ONE, 2014-07-10;9(7):e102180.
Applications: Bioassay -
Soft matrix is a natural stimulator for cellular invasiveness.
Authors: Gu, Zhizhan, Liu, Fei, Tonkova, Elina A, Lee, Soo Youn, Tschumperlin, Daniel J, Brenner, Michael
Mol Biol Cell, 2013-12-11;25(4):457-69.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Control -
Astrocytes directly influence tumor cell invasion and metastasis in vivo.
Authors: Wang, Ling, Cossette, Stephani, Rarick, Kevin R, Gershan, Jill, Dwinell, Michael, Harder, David R, Ramchandran, Ramani
PLoS ONE, 2013-12-04;8(12):e80933.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Bioassay -
Fibulin-3, -4, and -5 are highly susceptible to proteolysis, interact with cells and heparin, and form multimers.
Authors: Djokic J, Fagotto-Kaufmann C, Bartels R, Nelea V, Reinhardt D
J Biol Chem, 2013-06-19;288(31):22821-35.
Species: Human
Sample Types: Protein
Applications: Enzyme Assay -
Resistance of corneal RFUVA-cross-linked collagens and small leucine-rich proteoglycans to degradation by matrix metalloproteinases.
Authors: Zhang Y, Mao X, Schwend T, Littlechild S, Conrad G
Invest Ophthalmol Vis Sci, 2013-02-05;54(2):1014-25.
Species: Bovine
Sample Types: Protein
Applications: Enzyme Assay -
Simple pseudo-dipeptides with a P2' glutamate: a novel inhibitor family of matrix metalloproteases and other metzincins.
Authors: Devel L, Beau F, Amoura M, Vera L, Cassar-Lajeunesse E, Garcia S, Czarny B, Stura E, Dive V
J Biol Chem, 2012-06-11;287(32):26647-56.
Applications: Enzyme Assay -
Astacin proteases cleave dentin sialophosphoprotein (Dspp) to generate dentin phosphoprotein (Dpp).
Authors: Tsuchiya S, Simmer JP, Hu JC, Richardson AS, Yamakoshi F, Yamakoshi Y
J. Bone Miner. Res., 2011-01-01;26(0):220.
Species: Human
Sample Types:
Applications: Bioassay -
Development and validation of sandwich ELISA microarrays with minimal assay interference.
Authors: Gonzalez RM, Seurynck-Servoss SL, Crowley SA
J. Proteome Res., 2008-04-19;7(6):2406-14.
Applications: ELISA (Standard) -
Borrelia burgdorferi-induced monocyte chemoattractant protein-1 production in vivo and in vitro.
Authors: Zhao Z, McCloud B, Fleming R, Klempner MS
Biochem. Biophys. Res. Commun., 2007-05-02;358(2):528-33.
Applications: Zymography -
Role of platelet-derived growth factor and transforming growth factor beta1 the in the regulation of metalloproteinase expressions.
Authors: Borrelli V, di Marzo L, Sapienza P, Colasanti M, Moroni E, Cavallaro A
Surgery, 2006-09-01;140(3):454-63.
Applications: Western Blot -
Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer.
Authors: Zhou BB, Peyton M, He B, Liu C, Girard L, Caudler E, Lo Y, Baribaud F, Mikami I, Reguart N, Yang G, Li Y, Yao W, Vaddi K, Gazdar AF, Friedman SM, Jablons DM, Newton RC, Fridman JS, Minna JD, Scherle PA
Cancer Cell, 2006-07-01;10(1):39-50.
Species: Human
Sample Types:
Applications: Enzyme Assay -
Oxidized low-density lipoproteins stimulate extracellular matrix metalloproteinase Inducer (EMMPRIN) release by coronary smooth muscle cells.
Authors: Haug C, Lenz C, Diaz F, Bachem MG
Arterioscler. Thromb. Vasc. Biol., 2004-08-19;24(10):1823-9.
Species: Human
Sample Types: Protein
Applications: Bioassay
FAQs
-
Can the enzyme be stored after activation, or do I need to use it immediately after activation?
We recommend only activating the amount of enzyme needed for your assay, and recommend activating the enzyme immediately prior to use. Any unactivated enzyme should be stored in aliquots at either the stock concentration at which the enzyme was supplied, or the reconstitution concentration, according to the product datasheet.
-
Does this enzyme have a tag?
No, this enzyme does not have a tag. Please refer to the Source section on the product-specific page or product datasheet for sequence information.
-
If I use this enzyme at a higher concentration, do I need to change the concentration of APMA to activate it?
We have only optimized activation conditions for one particular concentration of this MMP enzyme as part of our regular QC testing for enzymatic activity. Activating the enzyme at any different concentration would have to be optimized by the end user.
-
Does this MMP enzyme need to be activated to work?
Yes, this enzyme requires activation prior to use.
-
What is the activity of this enzyme in units/µg?
We supply this enzyme as a mass and calculate its activity relative to mass (pmol/min/µg). We have not calibrated this enzyme to an international standard unit, so we are unable to provide a conversion to units/µg.
Reviews for Recombinant Human MMP-2 Protein, CF
Average Rating: 4.7 (Based on 3 Reviews)
Have you used Recombinant Human MMP-2 Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
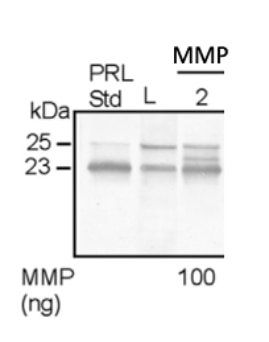
Reason for Rating: The product was very easy to dissolve and use in an enzymatic cleavage of proteins. The product showed an excellent cleavage activity.
rhMMP-2 was diluted up to a final concentration of 1 mM using p-amino phenylmercuric acetate (APMA) and mixed with chondrocyte lysate protein to test the cleavage of prolactin hormone (PRL) into 16K products. Western blot pictures showed the successful formation of several fragments after the mixture. L represents the Lysate, PRL Std the Prolactin Standard