Human/Mouse/Rat ERK1/ERK2 Antibody Summary
XM_055766 and NM_138957, respectively
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
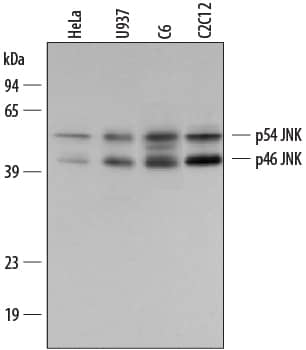
Detection of Human/Mouse/Rat ERK1/ERK2 by Western Blot. Western blot shows lysates of Jurkat human acute T cell leukemia cell line, C2C12 mouse myoblast cell line myoblast cell line, and C6 rat glioma cell line. PVDF membrane was probed with 0.2 µg/mL Rabbit Anti-Human/Mouse/Rat ERK1/ERK2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1576) followed by HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # HAF008). For additional reference, Recombinant Human Active ERK1 (Catalog # 1879-KS) and Recombinant Human Active ERK2 (Catalog # 1230-KS) (2 ng/lane) were included. A specific band for ERK1/ERK2 was detected at approximately 44 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of Mouse ERK1/ERK2 by Simple WesternTM. Simple Western lane view shows lysates of C2C12 mouse myoblast cell line, loaded at 0.2 mg/mL. A specific band was detected for ERK1/ERK2 at approximately 44 kDa (as indicated) using 10 µg/mL of Rabbit Anti-Human/Mouse/Rat ERK1/ERK2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1576). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot Phospho-proteome profiling results of LX-2 cells co-cultured with C3A–CYP2E1 cells with or without DDC treatment and detection of the effect of DDC on intracellular kinases in LX-2 cells of co-cultures200 μg of total cell lysates from LX-2 cells co-cultured with C3A-2E1 cells with or without 100 μM DDC for 1 h were incubated with membranes of the human phospho-MAPK Array Kit according to the manufacturer's instructions. Phospho MAPK Array data were developed on X-ray films following exposure to chemiluminescent reagents. 20 μg aliquots of total cell lysates from LX-2 cells were subjected to Western blotting analysis. (A) Template showing the location of MAPK antibodies spotted onto the human phospho-MAPK Array Kit. (B) The activation status of ERK1/2 in LX-2 cells co-cultured with C3A-2E1 cells after DDC treatment. (C) The activation status of p38 in LX-2 cells co-cultured with C3A-2E1 cells after DDC treatment. (D) The activation status of Akt in LX-2 cells co-cultured with C3A-2E1 cells after DDC treatment. (E) The activation status of ERK1/2, p38 and Akt in LX-2 cells co-cultured with C3A cells after DDC treatment. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot DDC up-regulates MMP-1 through ERK1/2 and Akt activation(A) Co-cultures were treated with 100 μM DDC for the indicated time. Phosphorylation of ERK1/2, p38 and Akt were determined by Western blotting analysis. The corresponding non-phosphorylated ERK1/2, p38, Akt and beta -actin were used for protein loading control. (B) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of ERK1/2 inhibitor (U0126, 10 μM). MMP-1 protein levels were analysed by Western blotting. (C) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of p38 inhibitor (SB203580, 10 μM). MMP-1 protein levels were analysed by Western blotting. (D) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of Akt inhibitor (T3830, 50 μM). MMP-1 protein levels were analysed by Western blotting. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot p38 inhibitor SB203580 improves the up-regulation of MMP-1 by DDC through stimulating ERK1/2Co-cultures were treated with or without 100 μM DDC for 1 h in the presence or absence of p38 inhibitor (SB203580, 10 μM). Phosphorylation of ERK1/2, p38 and Akt in LX-2 cells of co-cultures were determined by Western blotting analysis. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot Depletion of Rac1b increases constitutive and growth factor-induced ERK1/2 phosphorylation. (A) Panc1 cells were transiently transfected with siRNA specific to Rac1b (R1b) or irrelevant control siRNA (Co), serum-starved for 24 h and treated with TGF-beta 1 as indicated. Cells were subjected to immunoblotting for p-ERK1/2, t-ERK1/2 and HSP90 as well as Rac1b as a control for transfection efficiency. The chart below shows the relative intensities of the p-ERK1/2 bands normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). Data are displayed relative to non-stimulated Rac1b siRNA-transfected cells set arbitrarily at 1. Asterisks indicate a significant difference. (B) Colo357 and IMIM-PC1 cells were transiently transfected twice on two consecutive days with 50 nM each of a siRNA specific for Rac1b (R1b), or an irrelevant control siRNA (Co), serum-starved for 24 h and subjected to immunoblotting for phospho-ERK1/2 (p-ERK1/2) and total ERK1/2 (t-ERK1/2) as well al HSP90 as a loading control. The charts below the blots show the relative intensity of the p-ERK1/2 bands normalized to the t-ERK1/2 bands of three independent experiments (mean ± SD, n = 3). Asterisks indicate a significant difference. (C) Panc1 cells stably expressing a dominant negative ALK5 mutant (ALK5KR) or empty vector were transfected twice with 50 nM each of Co siRNA or Rac1b siRNA and subjected to immunoblot analysis of p-ERK1/2. The blot was stripped and reprobed with antibodies against and t-ERK1/2 and Rac1b. The graph below the blots show the relative intensities of p-ERK1/2 normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). (D) Panc1 cells were transfected with 50 nM of irrelevant Co siRNA, Rac1b siRNA or ALK5 siRNA, or 25 nM each of Rac1b + ALK5 siRNA. Cells were serum-starved, treated with TGF-beta 1 for 12 h and subjected to immunoblotting for p-ERK1/2, t-ERK1/2 and HSP90 as well as for Rac1b and ALK5 to verify successful depletion. The graph below the blots shows the relative intensities of p-ERK1/2 bands normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). Data are displayed relative to non-stimulated Rac1b siRNA-transfected cells set arbitrarily at 1. The asterisks indicate significance. Data displayed in each panel are from the same blot/gel treated subsequently with antibodies to p-ERK1/2, Rac1b, HSP90, and ALK5, then stripped and reincubated with an antibody to t-ERK1/2. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41598-017-15170-6), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot Depletion of Rac1b increases constitutive and growth factor-induced ERK1/2 phosphorylation. (A) Panc1 cells were transiently transfected with siRNA specific to Rac1b (R1b) or irrelevant control siRNA (Co), serum-starved for 24 h and treated with TGF-beta 1 as indicated. Cells were subjected to immunoblotting for p-ERK1/2, t-ERK1/2 and HSP90 as well as Rac1b as a control for transfection efficiency. The chart below shows the relative intensities of the p-ERK1/2 bands normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). Data are displayed relative to non-stimulated Rac1b siRNA-transfected cells set arbitrarily at 1. Asterisks indicate a significant difference. (B) Colo357 and IMIM-PC1 cells were transiently transfected twice on two consecutive days with 50 nM each of a siRNA specific for Rac1b (R1b), or an irrelevant control siRNA (Co), serum-starved for 24 h and subjected to immunoblotting for phospho-ERK1/2 (p-ERK1/2) and total ERK1/2 (t-ERK1/2) as well al HSP90 as a loading control. The charts below the blots show the relative intensity of the p-ERK1/2 bands normalized to the t-ERK1/2 bands of three independent experiments (mean ± SD, n = 3). Asterisks indicate a significant difference. (C) Panc1 cells stably expressing a dominant negative ALK5 mutant (ALK5KR) or empty vector were transfected twice with 50 nM each of Co siRNA or Rac1b siRNA and subjected to immunoblot analysis of p-ERK1/2. The blot was stripped and reprobed with antibodies against and t-ERK1/2 and Rac1b. The graph below the blots show the relative intensities of p-ERK1/2 normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). (D) Panc1 cells were transfected with 50 nM of irrelevant Co siRNA, Rac1b siRNA or ALK5 siRNA, or 25 nM each of Rac1b + ALK5 siRNA. Cells were serum-starved, treated with TGF-beta 1 for 12 h and subjected to immunoblotting for p-ERK1/2, t-ERK1/2 and HSP90 as well as for Rac1b and ALK5 to verify successful depletion. The graph below the blots shows the relative intensities of p-ERK1/2 bands normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). Data are displayed relative to non-stimulated Rac1b siRNA-transfected cells set arbitrarily at 1. The asterisks indicate significance. Data displayed in each panel are from the same blot/gel treated subsequently with antibodies to p-ERK1/2, Rac1b, HSP90, and ALK5, then stripped and reincubated with an antibody to t-ERK1/2. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41598-017-15170-6), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot Effects of ectopic overexpression of Rac1b on TGF-beta 1-induced ERK1/2 activation and gene expression. (A) Panc1 cells stably overexpressing Rac1b from the pCGN vector (two independent clones, #4 and #13) as well as empty-vector control cells (v) were serum-starved, treated with TGF-beta 1 for 12 h and subjected to immunoblotting for p-ERK1/2, t-ERK1/2, and HA-Rac1b. The graph underneath the blot shows results from densitometric analysis of the respective bands for p-ERK1/2 normalized to those for t-ERK1/2 and derived from four independent experiments (mean ± SD, n = 4). Data are displayed relative to TGF-beta 1 stimulated vector control cells set arbitrarily at 1. Asterisks indicate a significant difference of p-ERK1/2 band intensity between the vector control and the two HA-Rac1b expressing clones. Due to the short exposure time, endogenous Rac1b protein is not visible. (B) The same cells as in (A) were stimulated with TGF-beta 1 for 24 h and subjected to qPCR for E-cadherin and PAR2. Data are displayed as TGF-beta 1-treated over control cells and are the mean ± SD of 3 experiments. Asterisks indicate significant differences. Data are from the same blot/gel treated subsequently with antibodies to p-ERK1/2, Rac1b, and HSP90, then stripped and reincubated with an antibody to t-ERK1/2. The vertical lines between lanes 3 and 4 indicate removal of irrelevant lanes from the blot. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41598-017-15170-6), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot Depletion of Rac1b increases constitutive and growth factor-induced ERK1/2 phosphorylation. (A) Panc1 cells were transiently transfected with siRNA specific to Rac1b (R1b) or irrelevant control siRNA (Co), serum-starved for 24 h and treated with TGF-beta 1 as indicated. Cells were subjected to immunoblotting for p-ERK1/2, t-ERK1/2 and HSP90 as well as Rac1b as a control for transfection efficiency. The chart below shows the relative intensities of the p-ERK1/2 bands normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). Data are displayed relative to non-stimulated Rac1b siRNA-transfected cells set arbitrarily at 1. Asterisks indicate a significant difference. (B) Colo357 and IMIM-PC1 cells were transiently transfected twice on two consecutive days with 50 nM each of a siRNA specific for Rac1b (R1b), or an irrelevant control siRNA (Co), serum-starved for 24 h and subjected to immunoblotting for phospho-ERK1/2 (p-ERK1/2) and total ERK1/2 (t-ERK1/2) as well al HSP90 as a loading control. The charts below the blots show the relative intensity of the p-ERK1/2 bands normalized to the t-ERK1/2 bands of three independent experiments (mean ± SD, n = 3). Asterisks indicate a significant difference. (C) Panc1 cells stably expressing a dominant negative ALK5 mutant (ALK5KR) or empty vector were transfected twice with 50 nM each of Co siRNA or Rac1b siRNA and subjected to immunoblot analysis of p-ERK1/2. The blot was stripped and reprobed with antibodies against and t-ERK1/2 and Rac1b. The graph below the blots show the relative intensities of p-ERK1/2 normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). (D) Panc1 cells were transfected with 50 nM of irrelevant Co siRNA, Rac1b siRNA or ALK5 siRNA, or 25 nM each of Rac1b + ALK5 siRNA. Cells were serum-starved, treated with TGF-beta 1 for 12 h and subjected to immunoblotting for p-ERK1/2, t-ERK1/2 and HSP90 as well as for Rac1b and ALK5 to verify successful depletion. The graph below the blots shows the relative intensities of p-ERK1/2 bands normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). Data are displayed relative to non-stimulated Rac1b siRNA-transfected cells set arbitrarily at 1. The asterisks indicate significance. Data displayed in each panel are from the same blot/gel treated subsequently with antibodies to p-ERK1/2, Rac1b, HSP90, and ALK5, then stripped and reincubated with an antibody to t-ERK1/2. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41598-017-15170-6), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot Phospho-proteome profiling results of LX-2 cells co-cultured with C3A–CYP2E1 cells with or without DDC treatment and detection of the effect of DDC on intracellular kinases in LX-2 cells of co-cultures200 μg of total cell lysates from LX-2 cells co-cultured with C3A-2E1 cells with or without 100 μM DDC for 1 h were incubated with membranes of the human phospho-MAPK Array Kit according to the manufacturer's instructions. Phospho MAPK Array data were developed on X-ray films following exposure to chemiluminescent reagents. 20 μg aliquots of total cell lysates from LX-2 cells were subjected to Western blotting analysis. (A) Template showing the location of MAPK antibodies spotted onto the human phospho-MAPK Array Kit. (B) The activation status of ERK1/2 in LX-2 cells co-cultured with C3A-2E1 cells after DDC treatment. (C) The activation status of p38 in LX-2 cells co-cultured with C3A-2E1 cells after DDC treatment. (D) The activation status of Akt in LX-2 cells co-cultured with C3A-2E1 cells after DDC treatment. (E) The activation status of ERK1/2, p38 and Akt in LX-2 cells co-cultured with C3A cells after DDC treatment. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human ERK1/ERK2 by Western Blot Depletion of Rac1b increases constitutive and growth factor-induced ERK1/2 phosphorylation. (A) Panc1 cells were transiently transfected with siRNA specific to Rac1b (R1b) or irrelevant control siRNA (Co), serum-starved for 24 h and treated with TGF-beta 1 as indicated. Cells were subjected to immunoblotting for p-ERK1/2, t-ERK1/2 and HSP90 as well as Rac1b as a control for transfection efficiency. The chart below shows the relative intensities of the p-ERK1/2 bands normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). Data are displayed relative to non-stimulated Rac1b siRNA-transfected cells set arbitrarily at 1. Asterisks indicate a significant difference. (B) Colo357 and IMIM-PC1 cells were transiently transfected twice on two consecutive days with 50 nM each of a siRNA specific for Rac1b (R1b), or an irrelevant control siRNA (Co), serum-starved for 24 h and subjected to immunoblotting for phospho-ERK1/2 (p-ERK1/2) and total ERK1/2 (t-ERK1/2) as well al HSP90 as a loading control. The charts below the blots show the relative intensity of the p-ERK1/2 bands normalized to the t-ERK1/2 bands of three independent experiments (mean ± SD, n = 3). Asterisks indicate a significant difference. (C) Panc1 cells stably expressing a dominant negative ALK5 mutant (ALK5KR) or empty vector were transfected twice with 50 nM each of Co siRNA or Rac1b siRNA and subjected to immunoblot analysis of p-ERK1/2. The blot was stripped and reprobed with antibodies against and t-ERK1/2 and Rac1b. The graph below the blots show the relative intensities of p-ERK1/2 normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). (D) Panc1 cells were transfected with 50 nM of irrelevant Co siRNA, Rac1b siRNA or ALK5 siRNA, or 25 nM each of Rac1b + ALK5 siRNA. Cells were serum-starved, treated with TGF-beta 1 for 12 h and subjected to immunoblotting for p-ERK1/2, t-ERK1/2 and HSP90 as well as for Rac1b and ALK5 to verify successful depletion. The graph below the blots shows the relative intensities of p-ERK1/2 bands normalized to those for t-ERK1/2 from three independent experiments (mean ± SD, n = 3). Data are displayed relative to non-stimulated Rac1b siRNA-transfected cells set arbitrarily at 1. The asterisks indicate significance. Data displayed in each panel are from the same blot/gel treated subsequently with antibodies to p-ERK1/2, Rac1b, HSP90, and ALK5, then stripped and reincubated with an antibody to t-ERK1/2. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41598-017-15170-6), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
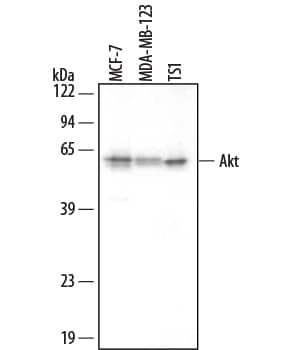
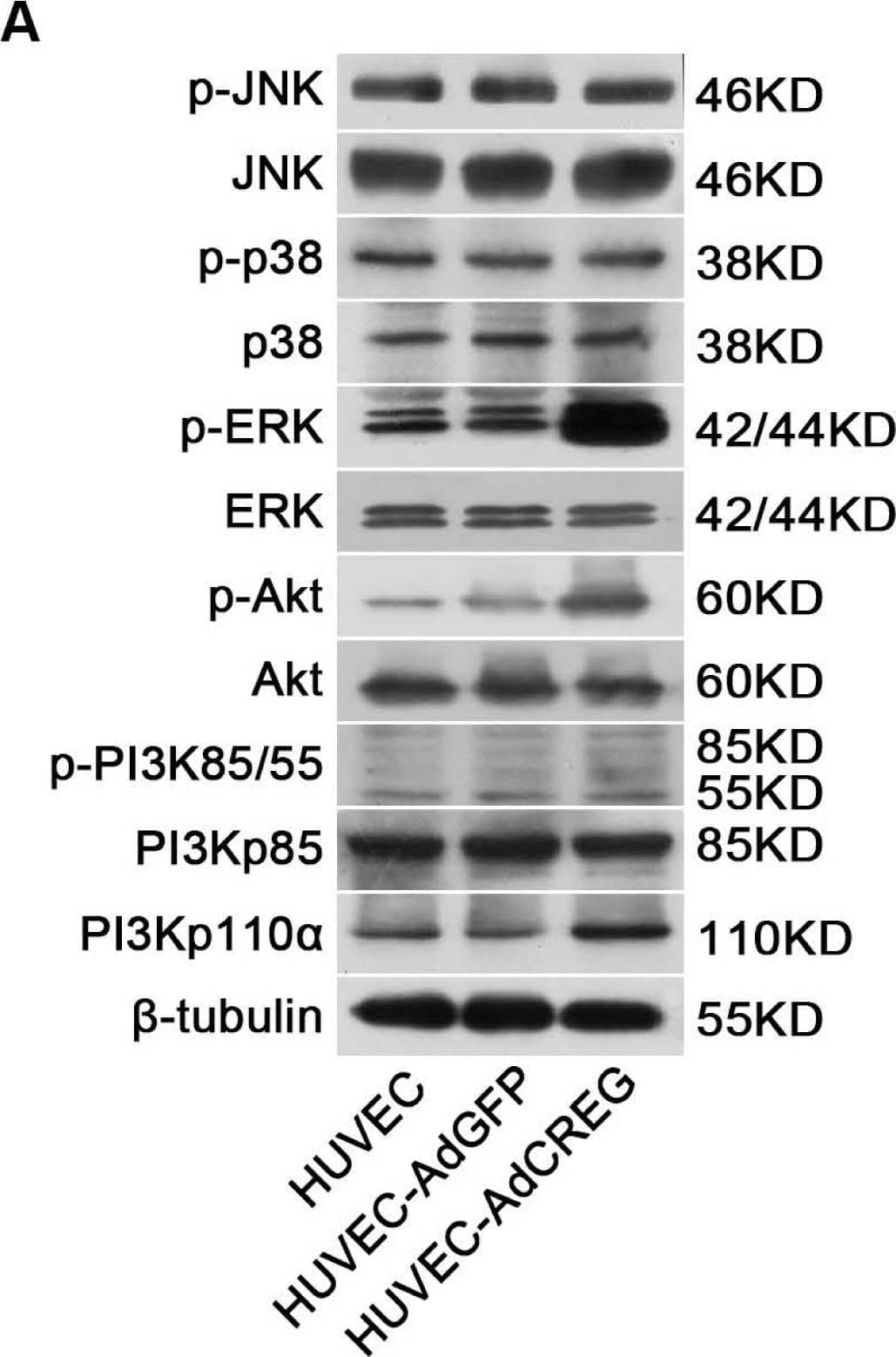
Detection of Human ERK1/ERK2 by Western Blot CREG overexpression activates both ERK&PI3K/Akt signaling pathways,&ERK, but not PI3K/Akt, mediates CREG effects on HUVEC proliferation. (A) In the three groups of cells (HUVEC, AdGFP&AdCREG), expression of JNK, p38, ERK, PI3K&Akt signaling molecules&their phosphorylated (p-) forms detected by WB, with beta -tubulin as the loading control. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/24018888), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
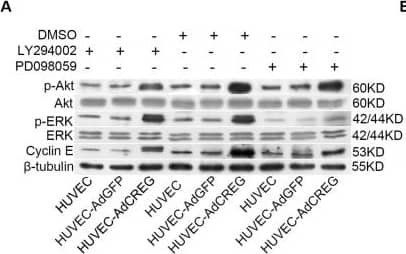
Detection of Human ERK1/ERK2 by Western Blot ERK, but not PI3K/Akt, mediates the increase of cyclin E induced by CREG overexpression in HUVEC. (A) Expression of p-ERK, ERK, p-Akt, Akt, cyclin E and beta -tubulin was detected by Western blotting in the three groups of cells with LY294002 (50 μM) or PD098059 (20 μM) treatment. Vehicle (DMSO)-treated cells were used as control, and beta -tubulin was used as a loading control; (B,C) Pooled analysis of the levels of Akt (B) and ERK (C) signaling activation accessed by the grayscale ratios of p-Akt/Akt and p-ERK/ERK, respectively. Data are given as the mean ± SD (n = 3). *p < 0.05; **p < 0.01. NS: no significant difference. (D) After normalization to beta -tubulin, the difference in the expression of cyclin E between the HUVEC-AdGFP and HUVEC-AdCREG group was reduced by the treatment of PD098059, but not LY294002. Data are given as the mean ± SD (n = 3). **p < 0.01. NS: no significant difference. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/24018888), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
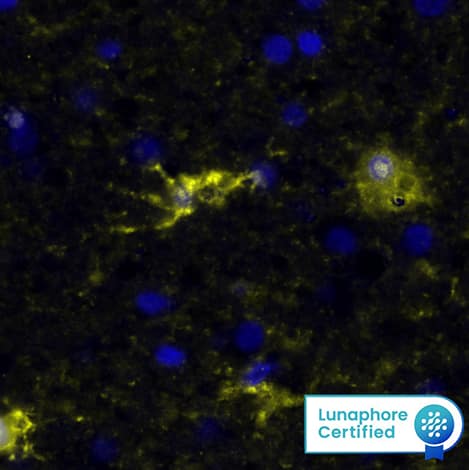
Detection of Human ERK1/ERK2 by Simple WesternTM. Image of total ERK in A549 cells. Primary antibody dilution - 1:20 in antibody diluent 2. 60 min of incubation. Image from a verified customer review.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: ERK1/ERK2
ERK1 and ERK2 (also known as MAPK3 and MAPK1) are 44 and 42 kDa Ser/Thr kinases, respectively. They are part of the Ras-Raf-ERK signal transduction cascade often found downstream of growth factor receptor activation. ERK1 and ERK2 were initially isolated and cloned as kinases activated in response to insulin and NGF. They are expressed in most, if not all, mammalian tissues. Dual threonine and tyrosine phosphorylation activate both ERKs, at Thr202/Tyr204 for human ERK1 and Thr185/Tyr187 for human ERK2.
ERK5, also known as Big Mitogen-activated Protein Kinase 1 (BMK1) and MAPK7, is activated by several mechanisms, including receptor tyrosine kinases, G protein-coupled receptors, and osmotic stress. Like ERK1 and ERK2, ERK5 contains the conserved Thr-Glu-Tyr activation motif in its activation loop. Unlike these ERKs, however, ERK5 contains a unique C-terminal domain that regulates its activation and nuclear translocation.
Product Datasheets
Citations for Human/Mouse/Rat ERK1/ERK2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
33
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
In vitro and in vivo anti-tumor activity of alectinib in tumor cells with NCOA4-RET
Authors: Sachiko Arai, Kenji Kita, Azusa Tanimoto, Shinji Takeuchi, Koji Fukuda, Hiroshi Sato et al.
Oncotarget
-
TGF-beta -Dependent Growth Arrest and Cell Migration in Benign and Malignant Breast Epithelial Cells Are Antagonistically Controlled by Rac1 and Rac1b
Authors: Catharina Melzer, Juliane von der Ohe, Ralf Hass, Hendrik Ungefroren
International Journal of Molecular Sciences
-
Extracellular signal‐regulated kinase regulates microglial immune responses in Alzheimer’s disease
Authors: Michael J. Chen, Supriya Ramesha, Laura D. Weinstock, Tianwen Gao, Lingyan Ping, Hailian Xiao et al.
Journal of Neuroscience Research
-
The miR-106b/NR2F2-AS1/PLEKHO2 Axis Regulates Migration and Invasion of Colorectal Cancer through the MAPK Pathway
Authors: Shuzhen Liu, Guoyan An, Qing Cao, Tong Li, Xinyu Jia, Lei Lei
International Journal of Molecular Sciences
-
Protective effect of puerarin against burn‑induced heart injury in rats
Authors: Junling Liu, Jianyun Liu, Mingming Bai, Hui Wang
Experimental and Therapeutic Medicine
-
MicroRNA‑193b acts as a tumor suppressor in colon cancer progression via targeting RAB22A
Authors: Zhiming Fang, Chengren Li, Shouchao Li
Experimental and Therapeutic Medicine
-
Succinate Mediates Tumorigenic Effects via Succinate Receptor 1: Potential for New Targeted Treatment Strategies in Succinate Dehydrogenase Deficient Paragangliomas
Authors: Dieter M. Matlac, Katerina Hadrava Vanova, Nicole Bechmann, Susan Richter, Julica Folberth, Hans K. Ghayee et al.
Front Endocrinol (Lausanne)
-
Suaeda glauca Attenuates Liver Fibrosis in Mice by Inhibiting TGF?1-Smad2/3 Signaling in Hepatic Stellate Cells
Authors: Hong, YJ;Kim, GH;Park, Y;Jo, HJ;Nam, MW;Kim, DG;Cho, H;Shim, HJ;Jin, JS;Rho, H;Han, CY;
Nutrients
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
The proprotein convertase furin regulates the development of thymic epithelial cells to ensure central immune tolerance
Authors: Liang Z, Zhang Z, Zhang Q et al.
iScience
-
Dose-Dependent Biphasic Action of Quetiapine on AMPK Signalling via 5-HT7 Receptor: Exploring Pathophysiology of Clinical and Adverse Effects of Quetiapine
Authors: M Okada, K Fukuyama, E Motomura
International Journal of Molecular Sciences, 2022-08-14;23(16):.
Species: Rat
Sample Types: Cell Lysates
Applications: Simple Western -
Brexpiprazole Reduces 5-HT7 Receptor Function on Astroglial Transmission Systems
Authors: K Fukuyama, E Motomura, M Okada
International Journal of Molecular Sciences, 2022-06-12;23(12):.
Species: Rat
Sample Types: Cell Lysates
Applications: Simple Western -
Brivaracetam and Levetiracetam Suppress Astroglial L-Glutamate Release through Hemichannel via Inhibition of Synaptic Vesicle Protein
Authors: K Fukuyama, M Okada
International Journal of Molecular Sciences, 2022-04-19;23(9):.
Species: Rat
Sample Types: Cell Lysates
Applications: Simple Western -
O-GlcNAcylation Is Essential for Rapid Pomc Expression and Cell Proliferation in Corticotropic Tumor Cells
Authors: Massman LJ, Pereckas M, Zwagerman NT, Olivier-Van Stichelen S.
Endocrinology
-
Distinct Effects of Escitalopram and Vortioxetine on Astroglial L-Glutamate Release Associated with Connexin43
Authors: T Shiroyama, K Fukuyama, M Okada
International Journal of Molecular Sciences, 2021-09-16;22(18):.
Species: Rat
Sample Types: Cell Lysates
Applications: Simple Western -
Autocrine TGF&beta1 Opposes Exogenous TGF&beta1-Induced Cell Migration and Growth Arrest through Sustainment of a Feed-Forward Loop Involving MEK-ERK Signaling
Authors: H Ungefroren, J Christl, C Eiden, UF Wellner, H Lehnert, JU Marquardt
Cancers, 2021-03-17;13(6):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
IL-24 deficiency protects mice against bleomycin-induced pulmonary fibrosis by repressing IL-4-induced M2 program in macrophages
Authors: LZ Rao, Y Wang, L Zhang, G Wu, L Zhang, FX Wang, LM Chen, F Sun, S Jia, S Zhang, Q Yu, JH Wei, HR Lei, T Yuan, J Li, X Huang, B Cheng, J Zhao, Y Xu, BW Mo, CY Wang, H Zhang
Cell Death Differ, 2020-11-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Age-Dependent and Sleep/Seizure-Induced Pathomechanisms of Autosomal Dominant Sleep-Related Hypermotor Epilepsy
Authors: Fukuyama K, Okada M.
International Journal of Molecular Sciences
-
DRD3 (dopamine receptor D3) but not DRD2 activates autophagy through MTORC1 inhibition preserving protein synthesis
Authors: P Barroso-Ch, D Luis-Ravel, F Fumagallo-, J Castro-Her, J Salas-Hern, J Rodriguez-, A Febles-Cas, I Cruz-Muros, D Afonso-Ora, P Abreu-Gonz, R Moratalla, MJ Millan, T Gonzalez-H
Autophagy, 2019-10-02;0(0):.
Species: Human, Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Characterization of TRKA signaling in acute myeloid leukemia
Authors: SM Herbrich, S Kannan, RM Nolo, M Hornbaker, J Chandra, PA Zweidler-M
Oncotarget, 2018-07-10;9(53):30092-30105.
Species: Human
Sample Types: Whole Cells
Applications: Western Blot -
Effect of electrical stimulation on neural regeneration via the p38-RhoA and ERK1/2-Bcl-2 pathways in spinal cord-injured rats
Authors: MC Joo, CH Jang, JT Park, SW Choi, S Ro, MS Kim, MY Lee
Neural Regen Res, 2018-02-01;13(2):340-346.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
The Role of PAR2 in TGF-?1-Induced ERK Activation and Cell Motility
Authors: H Ungefroren, D Witte, C Fiedler, T Gädeken, R Kaufmann, H Lehnert, F Gieseler, BH Rauch
Int J Mol Sci, 2017-12-20;18(12):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Negative regulation of TGF-?1-induced MKK6-p38 and MEK-ERK signalling and epithelial-mesenchymal transition by Rac1b
Authors: D Witte, H Otterbein, M Förster, K Giehl, R Zeiser, H Lehnert, H Ungefroren
Sci Rep, 2017-12-11;7(1):17313.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Obesity Increases Mitogen-Activated Protein Kinase Phosphatase-3 Levels in the Hypothalamus of Mice
Authors: BA Rodrigues, VR Muñoz, GK Kuga, RC Gaspar, SCBR Nakandakar, BM Crisol, JD Botezelli, LSS Pauli, ASR da Silva, LP de Moura, DE Cintra, ER Ropelle, JR Pauli
Front Cell Neurosci, 2017-10-09;11(0):313.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
TNF-alpha induces CXCL1 chemokine expression and release in human vascular endothelial cells in vitro via two distinct signaling pathways.
Authors: Lo H, Lai T, Li C, Wu W
Acta Pharmacol Sin, 2014-02-03;35(3):339-50.
Species: Human
Sample Types: N/A
-
A naturally occurring carotenoid, lutein, reduces PDGF and H2O2 signaling and compromised migration in cultured vascular smooth muscle cells.
Authors: Lo HM, Tsai YJ, Du WY, Tsou CJ, Wu WB
J. Biomed. Sci., 2012-02-08;19(0):18.
Species: Rat
Sample Types: Cell Lysates
Applications: Western Blot -
The EGFR ligands amphiregulin and heparin-binding egf-like growth factor promote peritoneal carcinomatosis in CXCR4-expressing gastric cancer.
Authors: Yasumoto K, Yamada T, Kawashima A, Wang W, Li Q, Donev IS, Tacheuchi S, Mouri H, Yamashita K, Ohtsubo K, Yano S
Clin. Cancer Res., 2011-04-11;17(11):3619-30.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Transient PI3K Inhibition Induces Apoptosis and Overcomes HGF-Mediated Resistance to EGFR-TKIs in EGFR Mutant Lung Cancer.
Authors: Donev IS, Wang W, Yamada T, Li Q, Takeuchi S, Matsumoto K, Yamori T, Nishioka Y, Sone S, Yano S
Clin. Cancer Res., 2011-01-10;17(8):2260-9.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion.
Authors: Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S
J. Mol. Cell. Cardiol., 2009-01-07;46(5):612-20.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
O-GlcNAcylation Is Essential for Rapid Pomc Expression and Cell Proliferation in Corticotropic Tumor Cells
Authors: Massman LJ, Pereckas M, Zwagerman NT, Olivier-Van Stichelen S.
Endocrinology
-
PKCalpha-induced drug resistance in pancreatic cancer cells is associated with transforming growth factor-beta1
Authors: Ying Chen, Guanzhen Yu, Danghui Yu, Minghua Zhu
Journal of Experimental & Clinical Cancer Research
-
Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury
Authors: Vincent Braunersreuther, Fabrizio Montecucco, Mohamed Asrih, Mohammed Ashri, Graziano Pelli, Katia Galan et al.
Journal of Molecular and Cellular Cardiology
-
Age-Dependent and Sleep/Seizure-Induced Pathomechanisms of Autosomal Dominant Sleep-Related Hypermotor Epilepsy
Authors: Fukuyama K, Okada M.
International Journal of Molecular Sciences
-
The proprotein convertase furin regulates the development of thymic epithelial cells to ensure central immune tolerance
Authors: Liang Z, Zhang Z, Zhang Q et al.
iScience
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsIsotype Controls
Reconstitution Buffers
Secondary Antibodies
Reviews for Human/Mouse/Rat ERK1/ERK2 Antibody
Average Rating: 5 (Based on 2 Reviews)
Have you used Human/Mouse/Rat ERK1/ERK2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
20 ug protein per sample
1:1000 dilution for Antibody.