IL-10 Cytokine Family Signaling Pathway
Click on one of the IL-10 family cytokines shown in the Explore Pathways box below to see a select list of cytokine-producing cells, major target cells, and the primary biological effects induced by each cytokine.
alpha
alpha
beta
beta
Dimer
Dimer
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
(Inactive)
(Inactive)
(Inactive)
(Inactive)
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Cell Proliferation
Cell Proliferation
alpha
alpha
beta
beta
Dimer
Dimer
alpha
alpha
beta
beta
alpha 1
alpha 1
beta
beta
Dimer
Dimer
alpha
alpha
beta
beta
alpha 1
alpha 1
beta
beta
Dimer
Dimer
alpha 1
alpha 1
beta
beta
Dimer
Dimer
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
(Inactive)
(Inactive)
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Cell Proliferation
Cell Proliferation
OR
Monomer Dimer
OR
Monomer Dimer
alpha
alpha
beta
beta
- Monocytes
- Macrophages
- Regulatory T cells
- T helper cells
- Regulatory B cells
- Natural killer cells
- Dendritic cells
- Granulocytes
- Monocytes
- Macrophages
- T cells
- B cells
- Natural killer cells
- Neutrophils
- Mast cells
- Dendritic cells
- Enhances the release of anti-inflammatory mediators by monocytes and macrophages
- Inhibits the release of pro-inflammatory mediators by monocytes, macrophages, neutrophils, eosinophils, and mast cells
- Inhibits the antigen presentation capacity of monocytes, macrophages, and dendritic cells
- Enhances the inhibitory, tolerance inducing, scavenger and phagocytic functions of monocytes/macrophages
- Inhibits proliferation and cytokine synthesis by CD4+ T cells
- Inhibits mast cell development
- Stimulates the proliferation, cytotoxic activity, and cytokine-producing capabilities of natural killer cells
- Enhances B cell proliferation, differentiation, and MHC class II expression
- IL-10 over-production is associated with undesired immunosuppressive effects and tumor growth
- IL-10 deficiency is associated with chronic inflammatory and autoimmune diseases including inflammatory bowel disease, psoriasis, and rheumatoid arthritis
- Monocytes
- Keratinocytes
- Macrophages
- B cells
- Airway epithelial cells
- Endothelial cells
- Fibroblasts
- Vascular smooth muscle cells
- Epithelial cells in the skin, lung, and reproductive organs
- Keratinocytes
- Monocytes
- T cells
- Endothelial cells
- Certain cancer cells
- Involved in skin wound healing
- Regulates inflammatory responses
- Has vasculoprotective effects
- Promotes Th2 polarization of naïve CD4+ T cells
- Pro-angiogenic: Stimulates endothelial cell proliferation, migration, and tube formation
- Monocytes
- Keratinocytes
- Dendritic cells
- Granulocytes
- Epithelial cells
- Endothelial cells
- Fibroblasts
- Epithelial cells in the skin, lung, and reproductive organs
- Keratinocytes
- Endothelial cells
- Synovial fibroblasts
- Certain cancer cells
- Regulates keratinocytes in a manner similar to IL-22 and IL-24
- Promotes the production of various anti-microbial proteins, pro-inflammatory mediators, chemokines, and angiogenesis factors
- Pro-angiogenic: Promotes endothelial cell proliferation, migration, and vascular tube formation
- May be involved in the pathogenesis of osteoporosis and some chronic inflammatory diseases including psoriasis, rheumatoid arthritis, inflammatory bowel disease, and atherosclerosis
- Th17 cells
- Th22 cells
- Th1 cells
- CD4+ Memory T cells
- Group 3 Innate lymphoid cells (ILC3s)
- CD8+ T cells
- gamma delta T cells
- Natural killer T cells
- Dendritic cells
- Neutrophils
- Bronchial and intestinal epithelial cells
- Keratinocytes
- Intestinal subepithelial myofibroblasts
- Hepatocytes
- Pancreatic acinar cells
- Certain cancer cells
- Regulates anti-microbial defense, migratory capacity, and terminal differentiation of keratinocytes
- Induces IL-20 production by keratinocytes
- Involved in skin wound healing
- Promotes the production of anti-microbial peptides and pro-inflammatory mediators by non-hematopoietic cells
- Promotes tissue protective and regenerative effects including maintenance of the epithelial barrier in the intestine and respiratory tracts, intestinal wound healing, hepatocyte protection during liver inflammation, protection against pancreatic tissue injury
- Capable of inducing an acute phase response
- Regulates metabolic pathways by inducing genes involved in lipid synthesis
- Promotes either protective or pathological responses in chronic inflammatory diseases depending on the cytokine milieu
- Elevated expression of IL-22, IL-22 deficiency, or mutations in IL-22 signaling pathway are associated with inflammatory and autoimmune diseases including psoriasis, inflammatory bowel disease, and rheumatoid arthritis
- Monocytes
- Th2 cells
- CD4+ Memory T cells
- Endothelial cells
- Epithelial cells
- Keratinocytes
- Melanocytes
- Colonic subepithelial myofibroblasts
- Epithelial cells in the skin, lung, and reproductive organs
- Keratinocytes
- Neutrophils
- Certain cancer cells
- Regulates keratinocytes in a manner similar to
IL-20 and IL-22 - Regulates expression of pro-inflammatory cytokines, anti-microbial peptides, and chemokines that promote the recruitment of leukocytes to epithelial tissue following infection
- Involved in skin wound healing
- Suppresses Wnt/beta-Catenin signaling and decreases the self renewal capabilities of stem cells
- Inhibits B cell maturation into plasma cells
- Has anti-inflammatory and protective effects on the intestinal mucosa
- Possesses anti-tumor activity: Promotes apoptosis and autophagy in cancer cells; Possesses immune stimulatory effects; Negatively regulates angiogenesis; Inhibits migration and invasion of cancer cells
- Increased levels in rheumatoid arthritis, inflammatory bowel disease, and psoriasis
- Th17 cells
- Memory T cells
- Natural killer cells
- Macrophages
- Fibroblasts
- Epithelial cells
- Keratinocytes
- Monocytes
- Neutrophils
- Dendritic cells
- Acts as an anti-microbial protein with direct bactericidal activity by forming pores in bacterial membranes that lead to cell lysis
- Exhibits priming effects on various immune cells to enhance anti-viral and anti-microbial responses
- Inhibits the proliferation of intestinal epithelial cells and promotes the production of pro-inflammatory cytokines
- Elevated expression found in autoimmune diseases such as psoriasis, rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease; SNPs in or near the IL-26 gene are associated with an increased risk of developing rheumatoid arthritis or ulcerative colitis

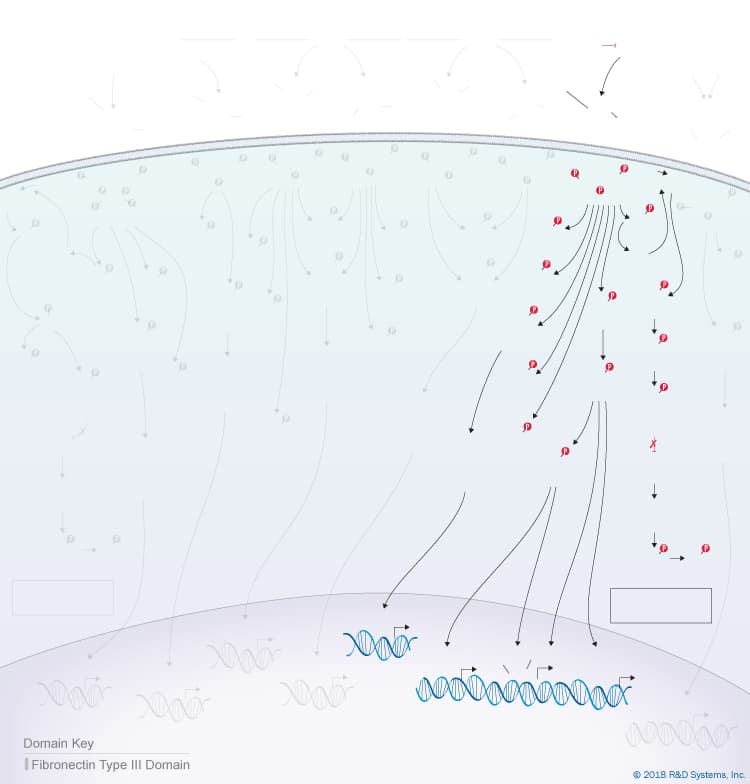
Overview of IL-10 Cytokine Family Signaling
The IL-10 family of cytokines consists of six members, IL-10, IL-19, IL-20, IL-22, IL-24, and IL-26, and is part of the larger class II cytokine family that also includes type I, type II, and type III interferons. Cytokines belonging to the IL-10 family are helical proteins that display relatively low amino acid sequence identity (between 9-40%) but have several unifying characteristics including similarities in their chromosomal locations, gene structures, protein secondary structures, and the heterodimeric receptor complexes that they utilize. All IL-10 family cytokines are compact molecules composed of six or seven alpha helices that form a hydrophobic core, which is stabilized by one to three disulfide bridges. While most members of the IL-10 family function as monomers, IL-10 forms a noncovalently-associated homodimer and IL-26, which has also been reported to have the capacity to form dimers, is thought to function as either a monomer or a dimer. Notably, there is no homologue of human IL-26 in mice. IL-10 family cytokines are secreted by a variety of immune and non-immune cell types. IL-10, IL-22, and IL-26 are primarily produced by different activated immune cell types, while IL-19, IL-20, and IL-24 can be secreted by both activated immune and non-immune cell types.
Monomeric members of the IL-10 cytokine family signal through heterodimeric class II cytokine receptors composed of one long chain transmembrane receptor (R1 chain) and one short chain transmembrane receptor (R2 chain), which are defined based on the lengths of their intracellular domains. Since IL-10 functions as a noncovalently-associated homodimer, it signals through a heterotetrameric receptor complex composed of two R1 chains and two R2 chains. Each of the six IL-10 cytokine family receptors contains one of three R1 chains, IL-10 R alpha/IL-10 R1, IL-20 R alpha/IL-20 R1, or IL-22 R alpha 1, and one of two R2 chains, IL-10 R beta/IL-10 R2 or IL-20 R beta/IL-20 R2, which all possess two tandem, extracellular fibronectin type III domains. Notably, all IL-10 family receptors share at least one chain with another receptor in the family. Adding to the complexity is the fact that some IL-10 family receptors can bind to more than one IL-10 family cytokine, and some IL-10 family cytokines can signal through more than one receptor complex. IL-10, IL-22, and IL-26 signal through receptor complexes that contain IL-10 R beta/IL-10 R2 paired with either IL-10 R alpha/IL-10 R1, IL-22 R alpha 1, or IL-20 R alpha/IL-20 R1, respectively. All three of these cytokines bind first to their respective R1 receptor chains and then recruit IL-10 R beta/IL-10 R2 to form the active receptor complex. In contrast, IL-19, IL-20, and IL-24 can all signal through the type I IL-20 receptor complex consisting of IL-20 R beta/IL-20 R2 paired with IL-20 R alpha/IL-20 R1, while both IL-20 and IL-24 can also bind with similar affinity and signal through a second type II IL-20 receptor complex consisting of IL-20 R beta/IL-20 R2 paired with IL-22 R alpha 1. IL-19, IL-20, and IL-24 initially interact with the R2 receptor chain (IL-20 R beta/IL-20 R2) and then recruit one of the R1 receptors to form an active receptor complex.
Following receptor binding, IL-10 family cytokines activate receptor-associated members of the Janus tyrosine kinase (Jak) protein family, leading to phosphorylation of the R1 chain of the receptor complex. Phosphorylation of the receptor creates a docking site for signal transducers and activators of transcription (STAT) family proteins, which are then phosphorylated by the Jak family kinases. Upon phosphorylation, the STAT proteins homodimerize or heterodimerize and translocate to the nucleus where they induce the expression of multiple target genes. STAT3 is the primary STAT activated by IL-10 family cytokines, although at higher ligand concentrations, several of these cytokines have also been shown to activate STAT1 and STAT5. In addition, both IL-10 and IL-22 activate PI 3-K/Akt signaling pathways, and both IL-22 and IL-20 have been shown to activate p38, JNK, and ERK1/2 mitogen-activated protein kinases (MAPKs) in multiple different cell types.
Despite similarities in their receptor complexes and the signaling pathways that they activate, IL-10 family cytokines have a variety of different biological effects due in part to the differential expression of their receptors. While immune cells are the main targets of IL-10, most resting and stimulated immune cells do not express IL-20 R alpha/IL-20 R1 or IL-22 R alpha 1. Instead, IL-20 R alpha/IL-20 R1 is preferentially expressed by tissue cells in the skin, lung, intestine, and reproductive organs, and IL-22 R alpha 1 is expressed by tissue cells in the skin, kidney, respiratory and digestive tracts. As a result, IL-10 has multiple effects on immune cells, while the other IL-10 family cytokines are primarily involved in regulating the activities of non-hematopoietic cell types.
In general, IL-10 has anti-inflammatory and immunosuppressive effects, and therefore plays a key role in downregulating immune responses and preventing excessive inflammation. The functions of monocytes and macrophages in particular are widely affected by IL-10. IL-10 enhances the release of anti-inflammatory mediators by these cells and inhibits their abilities to produce pro-inflammatory molecules. Additionally, IL-10 inhibits the antigen presentation capabilities of monocytes, macrophages, and dendritic cells, and enhances their tolerance-inducing, scavenger, and phagocytic functions. IL-10 can also suppress Th1-, Th2-, and Th17-mediated immune responses by inhibiting the proliferation of CD4+ T cells and their abilities to produce pro-inflammatory cytokines. Furthermore, it inhibits the secretion of pro-inflammatory mediators by neutrophils, eosinophils, and mast cells, along with mast cell development. In contrast to these immunosuppressive properties, IL-10 has also been shown to stimulate the proliferation and cytotoxic activity of natural killer cells, as well as the proliferation and cytolytic activity of CD8+ T cells in vitro in the presence of IL-2, and to enhance B cell proliferation, differentiation, and MHC class II expression. Overproduction of IL-10 has been shown to cause immunodeficiencies during infections or tumor growth, while a lack of IL-10 is associated with chronic inflammatory and autoimmune diseases including inflammatory bowel disease, psoriasis, and rheumatoid arthritis.
Unlike IL-10, the other IL-10 family cytokines seem to be primarily involved in regulating the activities of epithelial cells to promote tissue protective and regenerative processes aimed at maintaining tissue homeostasis. Similarities in the receptors, target cells, and functions of IL-19, IL-20, IL-22, IL-24, and IL-26 has led to their further categorization within the IL-10 family as IL-20 subfamily cytokines. All of these cytokines induce the expression of anti-microbial proteins in epithelial cells, except for IL-26 which functions directly as an anti-microbial protein itself. Most of these cytokines also promote epithelial cell expression of pro-inflammatory mediators and chemokines, which is important for the recruitment of leukocytes to sites of inflammation. IL-19, IL-22, and IL-24 are induced following skin injury in mice and humans, and IL-22 deficient mice display defects in skin wound healing, emphasizing the importance of these cytokines in the skin healing process. In addition, IL-20, IL-22, and IL-24 all have similar effects on keratinocytes, including their abilities to promote the secretion of anti-microbial proteins, stimulate keratinocyte proliferation and migration, and inhibit keratinocyte terminal differentiation. Notably, increased levels of IL-19, IL-20, IL-22, and IL-24 are found in psoriatic skin, indicating that they may be involved in the pathogenesis of this disease. Additional reports suggest that these cytokines may also be associated with the pathogenesis of other chronic inflammatory diseases including inflammatory bowel disease and rheumatoid arthritis. In addition to these activities, both IL-19 and IL-20 have been shown to have pro-angiogenic effects including the ability to induce endothelial cell proliferation, migration, and vascular tube formation. Although the precise function of IL-19 is not currently well understood, there is data to suggest that it has both vasculoprotective and anti-inflammatory effects. While the receptors for IL-20 subfamily cytokines are thought to be expressed primarily by non-hematopoietic cell types, IL-19 has also been shown to promote Th2 polarization of naïve CD4+ T cells, but the mechanism by which it does so is currently not clear.
Relative to the other IL-20 subfamily cytokines, the functions of IL-22 have been more widely studied. In addition to its previously described abilities to regulate keratinocyte functions, promote skin wound healing, and induce the expression of anti-microbial proteins and pro-inflammatory mediators in epithelial cells, IL-22 has also been shown to mediate protective effects on injured hepatocytes, pancreatic acinar cells, intestinal epithelial cells, and respiratory epithelial cells following K. pneumoniae infection. It has also been shown to have tissue regenerative effects in the liver, pancreas, kidneys, and thymus. Despite its tissue protective effects, IL-22 has also been shown to amplify the effects of some pro-inflammatory cytokines, and therefore, it has been reported to mediate protective or pathogenic effects depending on the affected tissue and the other cytokines present. Additionally, IL-22 has been suggested to regulate metabolic pathways by inducing the expression of genes involved in lipid synthesis. Notably, a natural soluble inhibitor of IL-22 known as IL-22BP has been identified. IL-22BP has a significantly higher affinity for IL-22 than IL-22 R alpha 1, suggesting that IL-22BP plays a central role in regulating the activity of IL-22. Elevated expression of IL-22, IL-22 deficiency, and mutations in the IL-22 signaling pathway have been associated with multiple inflammatory and autoimmune diseases including psoriasis, inflammatory bowel disease, and rheumatoid arthritis.
IL-24, also known as MDA-7 or FISP in mouse, was initially identified as one of the genes induced in terminally differentiating melanoma cells and was subsequently shown to have cancer cell-specific cytotoxic activity in human melanoma cells and multiple other cancer cell types. These effects were shown to be both receptor- and Jak/STAT signaling-independent and are the result of the ability of intracellular IL-24 to induce endoplasmic reticulum stress, generate reactive oxygen species, induce autophagy and apoptosis, and inhibit the migration of transformed cells. Besides these effects, IL-24 has also been shown to have receptor-dependent activities including the ability to induce the expression of pro-inflammatory cytokines, anti-microbial peptides, and chemokines that promote the recruitment of leukocytes to epithelial tissues in response to infection. Additionally, IL-24 has been suggested to promote wound healing, have anti-inflammatory and protective effects in the intestinal mucosa, have immune stimulatory effects in cancer, induce neutrophil chemotaxis in arthritis, promote keratinocyte activation, and be associated with the development of psoriasis.
IL-26, also known as AK155, is the final member of the IL-10 cytokine family. The functions of IL-26 are not currently well understood, but it has been characterized as a pro-inflammatory cytokine with anti-microbial activity. While other IL-20 subfamily cytokines promote the production of anti-microbial proteins, IL-26 has been found to have direct bactericidal activity. Additionally, IL-26 has been shown to stimulate the cytotoxic activity of natural killer cells against HCV-infected cells and to promote neutrophil chemotaxis to sites of infection. Elevated expression of IL-26 has been found in multiple inflammatory and autoimmune diseases including psoriasis, rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease, suggesting that it plays a role in the pathogenesis of these diseases. Additionally, single nucleotide polymorphisms (SNPs) in or near the IL-26 gene are associated with an increased risk of developing rheumatoid arthritis or ulcerative colitis.
To learn more, please visit our IL-10 Family Research Area page
Get Print Copy of this Pathway