Mouse ICAM-1/CD54 Antibody Summary
Gln28-Asn485
Accession # Q3U8M7
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
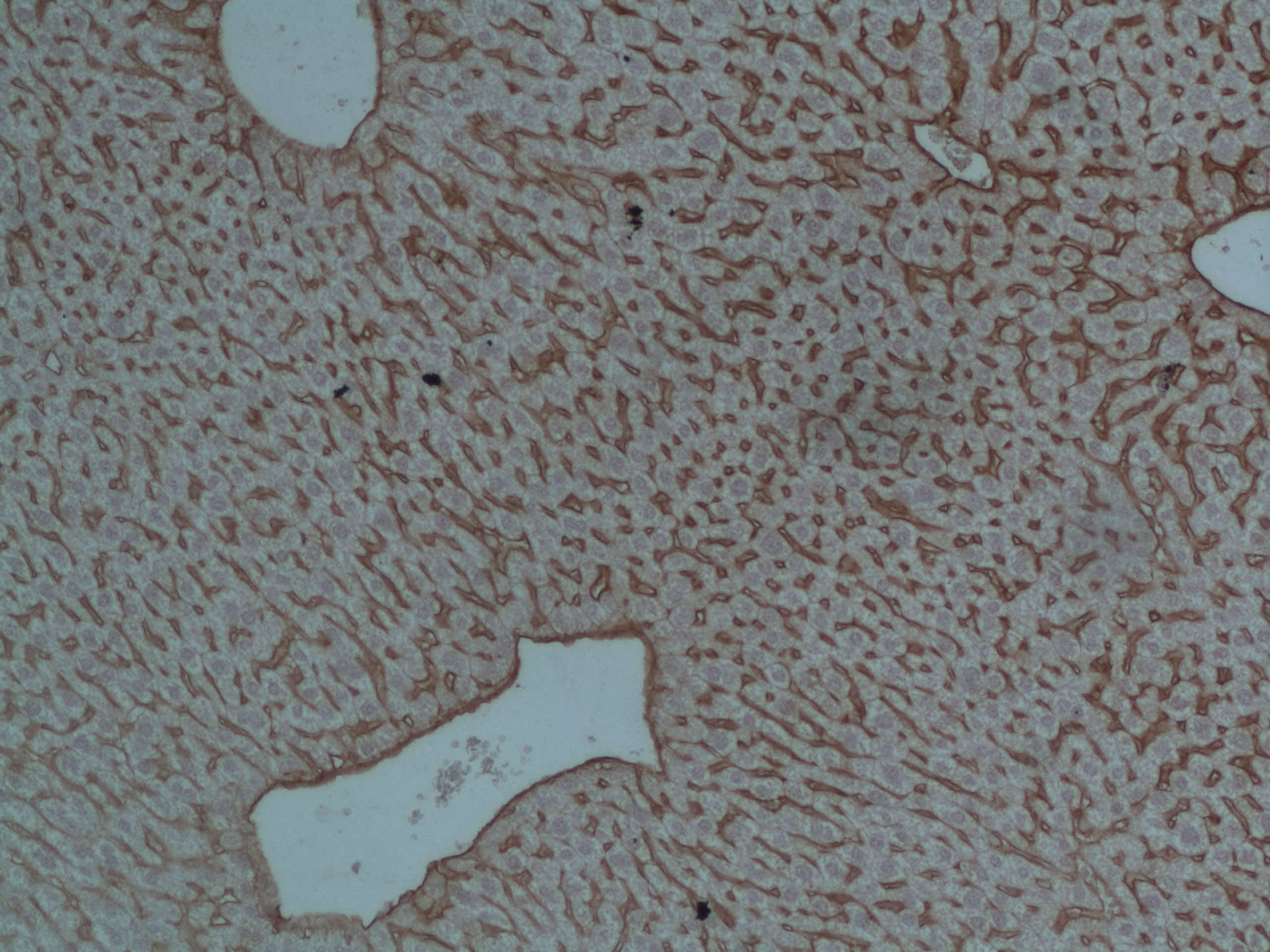
ICAM‑1/CD54 in Mouse Testis. ICAM-1/CD54 was detected in perfusion fixed frozen sections of mouse testis using Goat Anti-Mouse ICAM-1/CD54 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF796) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific staining was localized to plasma membrane in Leydig cells. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of Mouse ICAM-1/CD54 by Western Blot Increased stress kinase signaling and JNK pathway-dependent cytokine and chemokine production by primary keratinocytes lacking BRAF and RAF1.(A) Reduced ERK phosphorylation and increased JNK/p38 activation in primary delta / delta ep2 keratinocytes stimulated with EGF and/or TNF alpha and IL1 beta for 15 min. (B) Increased cytokine and chemokine production in primary delta / delta ep2 keratinocytes treated with EGF, TNF alpha and IL1 beta for 24 hr. Cytokine and chemokine production was determined by multiplex analysis, except for TSLP which was quantified by ELISA. Data represent mean ± SEM of 3–5 biological replicates. (C–D) Cells were pretreated with D-JNKI1 inhibitors prior to stimulation with EGF, TNF alpha and IL1 beta for 15 min (C) or 24 hr (D). Data represent the mean ± SEM of technical replicates (n = 3). (E–F) Effect of shRNA-mediated Mlk3 silencing on ERK and JNK phosphorylation and ICAM1 expression (E; stimulation with EGF, TNF alpha and IL1 beta for 15 min) and on the expression of Ccl2 and Tslp mRNA (F; stimulation with EGF, TNF alpha and IL1 beta for 24 hr) by F/F2 and delta / delta ep2 keratinocytes. shRen, shRNA targeting Renilla, used as a control; sh1 and sh2, targeting Mlk3, binding sites nucleotide 2266–2285 and 2383–2402, respectively. The shRNAs were encoded by lentiviral vectors coexpressing GFP. GFP immunoblots are shown to confirm similar levels of infection in all samples. Data represent mean ± SEM of 4 biological replicates. Each keratinocyte culture represents a pool of three mice. Immunoblots are representative of three independent experiments. p1 = 0.041, p2 = 0.040, p3 = 1.89E-4, p4 = 0.018, p5 = 0.046, p6 = 0.020, p7 = 0.008, p8 = 0.016, p9 = 0.001, p10 = 0.018, p11 = 3.23E-4, p12 = 1.47E-4, p13 = 0.007, p14 = 0.03, p15 = 0.035, p16 = 0.023 and p17 = 0.046.DOI:https://dx.doi.org/10.7554/eLife.14012.018Compound knockdown (KD2) of BRAF and RAF1 induce the expression of inflammation markers by HaCat cells in a MLK3/JNK-dependent manner.(A) Reduced ERK and increased JNK/p38 activation in BRAF and RAF1 knockdown (KD2) HaCat cells stimulated with EGF, TNF alpha and IL1 beta for 15 min. (B) D-JNKI1 reduces ICAM1 and CCL2 (n = 4) expression in KD2 cells treated with TNF alpha. (C) MEKi induces ICAM1 and CCL2 (n = 3) expression in RAF1KD cells treated with TNF alpha. In (B–C), ICAM1 expression was measured after a 3 hr, CCL2 expression after a 24 hr treatment with TNF alpha. (D) Effect of MLK3 silencing on ERK and JNK phosphorylation in WT and KD2 cells stimulated as in (A). MLK3 was silenced using a pool of oligonucleotides targeting the following regions: 686–704; 1489–1507; 2122–2138; and 2348–2366. MLK3 KD cells stimulated as in (B–C) show a decrease in JNK activation, ICAM1 and CCL2 (n = 7) expression. Immunoblots are representative of three independent experiments. qPCR data represent mean ± SEM of three independent experiments run in duplicates (p1 = 4.62E-4, p2 = 0.013, p3 = 0.050, p4 = 8.60E-8, p5 = 0.050, p6 = 0.001, p7 = 0.001 and p8 = 0.012).DOI:https://dx.doi.org/10.7554/eLife.14012.019 Image collected and cropped by CiteAb from the following publication (https://elifesciences.org/articles/14012), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ICAM-1/CD54 by Western Blot Increased stress kinase signaling and JNK pathway-dependent cytokine and chemokine production by primary keratinocytes lacking BRAF and RAF1.(A) Reduced ERK phosphorylation and increased JNK/p38 activation in primary delta / delta ep2 keratinocytes stimulated with EGF and/or TNF alpha and IL1 beta for 15 min. (B) Increased cytokine and chemokine production in primary delta / delta ep2 keratinocytes treated with EGF, TNF alpha and IL1 beta for 24 hr. Cytokine and chemokine production was determined by multiplex analysis, except for TSLP which was quantified by ELISA. Data represent mean ± SEM of 3–5 biological replicates. (C–D) Cells were pretreated with D-JNKI1 inhibitors prior to stimulation with EGF, TNF alpha and IL1 beta for 15 min (C) or 24 hr (D). Data represent the mean ± SEM of technical replicates (n = 3). (E–F) Effect of shRNA-mediated Mlk3 silencing on ERK and JNK phosphorylation and ICAM1 expression (E; stimulation with EGF, TNF alpha and IL1 beta for 15 min) and on the expression of Ccl2 and Tslp mRNA (F; stimulation with EGF, TNF alpha and IL1 beta for 24 hr) by F/F2 and delta / delta ep2 keratinocytes. shRen, shRNA targeting Renilla, used as a control; sh1 and sh2, targeting Mlk3, binding sites nucleotide 2266–2285 and 2383–2402, respectively. The shRNAs were encoded by lentiviral vectors coexpressing GFP. GFP immunoblots are shown to confirm similar levels of infection in all samples. Data represent mean ± SEM of 4 biological replicates. Each keratinocyte culture represents a pool of three mice. Immunoblots are representative of three independent experiments. p1 = 0.041, p2 = 0.040, p3 = 1.89E-4, p4 = 0.018, p5 = 0.046, p6 = 0.020, p7 = 0.008, p8 = 0.016, p9 = 0.001, p10 = 0.018, p11 = 3.23E-4, p12 = 1.47E-4, p13 = 0.007, p14 = 0.03, p15 = 0.035, p16 = 0.023 and p17 = 0.046.DOI:https://dx.doi.org/10.7554/eLife.14012.018Compound knockdown (KD2) of BRAF and RAF1 induce the expression of inflammation markers by HaCat cells in a MLK3/JNK-dependent manner.(A) Reduced ERK and increased JNK/p38 activation in BRAF and RAF1 knockdown (KD2) HaCat cells stimulated with EGF, TNF alpha and IL1 beta for 15 min. (B) D-JNKI1 reduces ICAM1 and CCL2 (n = 4) expression in KD2 cells treated with TNF alpha. (C) MEKi induces ICAM1 and CCL2 (n = 3) expression in RAF1KD cells treated with TNF alpha. In (B–C), ICAM1 expression was measured after a 3 hr, CCL2 expression after a 24 hr treatment with TNF alpha. (D) Effect of MLK3 silencing on ERK and JNK phosphorylation in WT and KD2 cells stimulated as in (A). MLK3 was silenced using a pool of oligonucleotides targeting the following regions: 686–704; 1489–1507; 2122–2138; and 2348–2366. MLK3 KD cells stimulated as in (B–C) show a decrease in JNK activation, ICAM1 and CCL2 (n = 7) expression. Immunoblots are representative of three independent experiments. qPCR data represent mean ± SEM of three independent experiments run in duplicates (p1 = 4.62E-4, p2 = 0.013, p3 = 0.050, p4 = 8.60E-8, p5 = 0.050, p6 = 0.001, p7 = 0.001 and p8 = 0.012).DOI:https://dx.doi.org/10.7554/eLife.14012.019 Image collected and cropped by CiteAb from the following publication (https://elifesciences.org/articles/14012), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ICAM-1/CD54 by Western Blot Increased stress kinase signaling and JNK pathway-dependent cytokine and chemokine production by primary keratinocytes lacking BRAF and RAF1.(A) Reduced ERK phosphorylation and increased JNK/p38 activation in primary delta / delta ep2 keratinocytes stimulated with EGF and/or TNF alpha and IL1 beta for 15 min. (B) Increased cytokine and chemokine production in primary delta / delta ep2 keratinocytes treated with EGF, TNF alpha and IL1 beta for 24 hr. Cytokine and chemokine production was determined by multiplex analysis, except for TSLP which was quantified by ELISA. Data represent mean ± SEM of 3–5 biological replicates. (C–D) Cells were pretreated with D-JNKI1 inhibitors prior to stimulation with EGF, TNF alpha and IL1 beta for 15 min (C) or 24 hr (D). Data represent the mean ± SEM of technical replicates (n = 3). (E–F) Effect of shRNA-mediated Mlk3 silencing on ERK and JNK phosphorylation and ICAM1 expression (E; stimulation with EGF, TNF alpha and IL1 beta for 15 min) and on the expression of Ccl2 and Tslp mRNA (F; stimulation with EGF, TNF alpha and IL1 beta for 24 hr) by F/F2 and delta / delta ep2 keratinocytes. shRen, shRNA targeting Renilla, used as a control; sh1 and sh2, targeting Mlk3, binding sites nucleotide 2266–2285 and 2383–2402, respectively. The shRNAs were encoded by lentiviral vectors coexpressing GFP. GFP immunoblots are shown to confirm similar levels of infection in all samples. Data represent mean ± SEM of 4 biological replicates. Each keratinocyte culture represents a pool of three mice. Immunoblots are representative of three independent experiments. p1 = 0.041, p2 = 0.040, p3 = 1.89E-4, p4 = 0.018, p5 = 0.046, p6 = 0.020, p7 = 0.008, p8 = 0.016, p9 = 0.001, p10 = 0.018, p11 = 3.23E-4, p12 = 1.47E-4, p13 = 0.007, p14 = 0.03, p15 = 0.035, p16 = 0.023 and p17 = 0.046.DOI:https://dx.doi.org/10.7554/eLife.14012.018Compound knockdown (KD2) of BRAF and RAF1 induce the expression of inflammation markers by HaCat cells in a MLK3/JNK-dependent manner.(A) Reduced ERK and increased JNK/p38 activation in BRAF and RAF1 knockdown (KD2) HaCat cells stimulated with EGF, TNF alpha and IL1 beta for 15 min. (B) D-JNKI1 reduces ICAM1 and CCL2 (n = 4) expression in KD2 cells treated with TNF alpha. (C) MEKi induces ICAM1 and CCL2 (n = 3) expression in RAF1KD cells treated with TNF alpha. In (B–C), ICAM1 expression was measured after a 3 hr, CCL2 expression after a 24 hr treatment with TNF alpha. (D) Effect of MLK3 silencing on ERK and JNK phosphorylation in WT and KD2 cells stimulated as in (A). MLK3 was silenced using a pool of oligonucleotides targeting the following regions: 686–704; 1489–1507; 2122–2138; and 2348–2366. MLK3 KD cells stimulated as in (B–C) show a decrease in JNK activation, ICAM1 and CCL2 (n = 7) expression. Immunoblots are representative of three independent experiments. qPCR data represent mean ± SEM of three independent experiments run in duplicates (p1 = 4.62E-4, p2 = 0.013, p3 = 0.050, p4 = 8.60E-8, p5 = 0.050, p6 = 0.001, p7 = 0.001 and p8 = 0.012).DOI:https://dx.doi.org/10.7554/eLife.14012.019 Image collected and cropped by CiteAb from the following publication (https://elifesciences.org/articles/14012), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ICAM-1/CD54 by Western Blot D-JNKI1 treatment rescues inflammation in delta / delta ep2 mice.Mice were treated with D-JNKI1 or TAT peptide (22 mg/kg i.p. at 10 days of age) and analyzed after 12 days (A) D-JNKI1 treatment prevents disease onset in delta / delta ep2 mice. Immunoblot of epidermal lysates showing the effect of D-JNKI1 on the phosphorylation and expression of the indicated proteins, quantified as in Figure 1F. ACTB is shown as a loading control. (B–D) Decreased eyelid inflammation, mast cells infiltration (B; TB+; quantified in the plot on the right), epidermal chemokine/cytokine levels (C) and activated T cells, B cells and dendritic cells in lymph nodes (D) in D-JNKI1-treated delta / delta ep2 mice. Scale bars, 25 µm. Data represent mean ± SEM (n = 3–5; p1 = 0.026, p2 = 0.042, p3 = 0.022, p4 = 0.048, p5 = 0.044, p6 = 0.020, p7 = 0.025, p8 = 0.018, p9 = 0.016, p10 = 0.014, p11 = 0.023, p12 = 0.011, p13 = 0.039, p14 = 0.049, p15 = 0.015, p16 = 0.003, p17 = 1.70E-4, p18 = 0.008, p19 = 0.008, p20 = 0.004, p21 = 0.003, p22 = 0.017, p23 = 0.026, p24 = 0.027, p25 = 0.005, p26 = 2.13E-6, p27 = 4.50E-8, p28 = 1.39E-5, p29 = 0.001, p30 = 0.001, p31 = 0.001, p32 = 0.023, p33 = 2.35E-4, p34 = 0.050, p35 = 0.002, p36 = 0.050 and p37 = 0.012).DOI:https://dx.doi.org/10.7554/eLife.14012.015K6 expression and epidermal chemokine and cytokine levels in D-JNKI1-treated mice.(A) K6 expression is indistinguishable in TAT or D-JNKI1 treated F/F2 and △/△ep2 littermates. Scale bars, 25 µm. (B) Inflammatory chemokines and cytokines in epidermal lysates of TAT or D-JNKI1-treated mice. Data represent mean ± SEM (n = 3–5; p1 = 0.005, p2 = 0.001, p3 = 0.043, p4 = 0.026, p5 = 0.032, p6 = 0.016 and p7 = 0.051).DOI:https://dx.doi.org/10.7554/eLife.14012.016The inflammatory phenotype of delta / delta ep2 mice is not rescued by MyD88, caspase 1/11, or TNF knockout.Macroscopic appearance, spleen and lymph node size and circulating blood cell analysis are shown for the indicated genotypes (A–C). (A) Representative pictures of 4 month old delta / delta ep2, delta / delta ep2 MyD88-/- and control animals. Plots on the right represent the ratio between total splenocytes or lymph node cell numbers and body weight (n = 3–4). (B) Representative pictures and hemogram of 4 month old delta / delta ep2, delta / delta ep2 caspase 1/11-/- and control animals (n = 5–6). (C) Representative pictures and hemogram of 4 month old delta / delta ep2, delta / delta ep2 TNF-/- and control animals (n = 4–5). The macroscopic appearance of at least ten mice per genotype was monitored. Data represent mean ± SEM. p1 = 0.041, p2 = 0.052, p3 = 0.024, p4 = 0.023, p5 = 0.026, p6 = 0.034, p7 = 0.023, p8 = 0.007, p9 = 0.031, p10 = 0.011, and p11 = 0.034.DOI:https://dx.doi.org/10.7554/eLife.14012.017 Image collected and cropped by CiteAb from the following publication (https://elifesciences.org/articles/14012), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
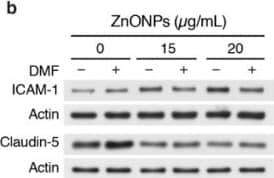
Detection of Human ICAM-1/CD54 by Western Blot Aryl hydrocarbon receptor (AhR) inhibitor 3′,4′-dimethoxyflavone (DMF) abolishes ZnONPs-induced ICAM-1 but not claudin-5 expressions or alters VE-cadherin junctional distribution. HUVECs were pretreated with 10 µM DMF, before treatment with ZnONPs for 24 h. (a) Representative images of immunofluorescence staining with antibody against VE-cadherin c-terminal domain (red) are shown. Cell nuclei were stained with DAPI (blue). *, diffused cytosolic distribution of VE-cadherin. (b) Western blotting was performed to detect ICAM-1 and claudin-5 expressions. Actin was used as a loading control. A representative of at least four experiments is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/32414036), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: ICAM-1/CD54
Intercellular Adhesion Molecule-1 (ICAM-1, CD54) binds the leukocyte integrins LFA-1 and Mac-1. ICAM-1 expression is weak on leukocytes, epithelial and resting endothelial cells, as well as some other cell types, but expression can be stimulated by IFN-gamma, TNF-alpha, IL-1 beta, and LPS. Mouse and human ICAM-1 share approximately 54% amino acid identity.
Soluble ICAM-1 is found in a biologically active form in serum, probably as a result of proteolytic cleavage from the cell surface, and is elevated in patients with various inflammatory syndromes such as septic shock, leukocyte adhesion deficiency syndrome (LAD), cancer, and transplantation.
- Pigott, R. and C. Power (1993) in The Adhesion Molecule Facts Book. Academic Press, p. 74.
- Siu, G. et al. (1989) J. Immunol. 143:3813.
- Ballantyne, C.M. et al. (1989) Nuc. Acid. Res. 17:5853.
Product Datasheets
Citations for Mouse ICAM-1/CD54 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
89
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Endothelial Msx1 transduces hemodynamic changes into an arteriogenic remodeling response
Authors: Ine Vandersmissen, Sander Craps, Maarten Depypere, Giulia Coppiello, Nick van Gastel, Frederik Maes et al.
Journal of Cell Biology
-
ARID1A and PI3-kinase pathway mutations in the endometrium drive epithelial transdifferentiation and collective invasion
Authors: Mike R. Wilson, Jake J. Reske, Jeanne Holladay, Genna E. Wilber, Mary Rhodes, Julie Koeman et al.
Nature Communications
-
Apolipoprotein E receptor 2 deficiency decreases endothelial adhesion of monocytes and protects against autoimmune encephalomyelitis
Authors: Calvier L, Manouchehri N, Sacharidou A et al.
Science Immunology
-
Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain
Authors: Caroline M. Sawicki, Daniel B. McKim, Eric S. Wohleb, Brant L. Jarrett, Brenda F. Reader, Diana M. Norden et al.
Neuroscience
-
Lumbar Myeloid Cell Trafficking into Locomotor Networks after Thoracic Spinal Cord Injury
Authors: Christopher N. Hansen, Diana M. Norden, Timothy D. Faw, Rochelle Deibert, Eric S. S.Wohleb, John F. Sheridan et al.
Experimental Neurology
-
Inhibition of the Akt1-mTORC1 Axis Alters Venous Remodeling to Improve Arteriovenous Fistula Patency.
Authors: Guo Xiangjiang, Fereydooni Arash, Isaji Toshihiko et al.
Scientific Reports
-
Infiltration of peripheral immune cells into the olfactory bulb in a mouse model of acute nasal inflammation
Authors: Hinami Asano, Sanae Hasegawa-Ishii, Ken Arae, Aki Obara, Geoffroy Laumet, Robert Dantzer et al.
Journal of Neuroimmunology
-
CAF1-knockout mice are more susceptive to lipopolysaccharide-induced acute lung injury
Authors: Jia-Xin Shi, Jia-Shu Li, Rong Hu, Xiao-Min Li, Hong Wang
Journal of Inflammation Research
-
Effect of Rosiglitazone on Liver Structure and Function in Genetically Diabetic Akita Mice
Authors: Bianca Hemmeryckx, Marijke Gaekens, David J. Gallacher, Hua Rong Lu, Henri Roger Lijnen
Basic & Clinical Pharmacology & Toxicology
-
Aedes aegypti salivary gland extract alleviates acute itching by blocking TRPA1 channels
Authors: Anderson R. A. Cerqueira, Leandro Rodrigues, Silvia Abigail Coavoy-Sánchez, Simone A. Teixeira, Karla B. Feitosa, Erika Y. Taniguchi et al.
Frontiers in Physiology
-
Matrix metalloproteinase-9 in the initial injury after hepatectomy in mice
Authors: Norifumi Ohashi, Tomohide Hori, Florence Chen, Sura Jermanus, Akimasa Nakao, Shinji Uemoto et al.
World Journal of Gastroenterology
-
Antiphospholipid antibodies induce proinflammatory and procoagulant pathways in endothelial cells
Authors: Markos Patsouras, Eirini Alexopoulou, Spyros Foutadakis, Eirini Tsiki, Panagiota Karagianni, Marios Agelopoulos et al.
Journal of Translational Autoimmunity
-
Reelin Depletion Protects Against Atherosclerosis by Decreasing Vascular Adhesion of Leukocytes
Authors: Laurent Calvier, Xunde Xian, Richard G. Lee, Anastasia Sacharidou, Chieko Mineo, Philip W. Shaul et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer
Authors: Marcus Ruscetti, John P. Morris, Riccardo Mezzadra, James Russell, Josef Leibold, Paul B. Romesser et al.
Cell
-
STAT3/NF‑ kappa B decoy oligodeoxynucleotides inhibit atherosclerosis through regulation of the STAT/NF‑ kappa B signaling pathway in a mouse model of atherosclerosis
Authors: Hyun-Jin An, Mi-Gyeong Gwon, Hyemin Gu, Seongjae Bae, Jaechan Leem, Jin Bae Lee et al.
International Journal of Molecular Medicine
-
Group 2 Innate Lymphoid Cells Protect Mice from Abdominal Aortic Aneurysm Formation via IL5 and Eosinophils
Authors: Yuanyuan Zhang, Tianxiao Liu, Zhiyong Deng, Wenqian Fang, Xian Zhang, Shuya Zhang et al.
Advanced Science
-
Single-cell transcriptomic atlas of taste papilla aging
Authors: Ren, W;Li, W;Cha, X;Wang, S;Cai, B;Wang, T;Li, F;Li, T;Xie, Y;Xu, Z;Wang, Z;Liu, H;Yu, Y;
Aging cell
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Characterization of focused ultrasound blood-brain barrier disruption effect on inflammation as a function of treatment parameters
Authors: Angolano, C;Hansen, E;Ajjawi, H;Nowlin, P;Zhang, Y;Thunemann, N;Ferran, C;Todd, N;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: Immunohistochemistry, Western Blot -
Kupffer Cell Inactivation Alters Endothelial Cell Adhesion Molecules in Cecal Ligation and Puncture-Induced Sepsis
Authors: Manandhar, S;Gaddam, RR;Chambers, S;Bhatia, M;
Biomolecules
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neural precursor cell delivery induces acute post-ischemic cerebroprotection, but fails to promote long-term stroke recovery in hyperlipidemic mice due to mechanisms that include pro-inflammatory responses associated with brain hemorrhages
Authors: Yin, D;Wang, C;Qi, Y;Wang, YC;Hagemann, N;Mohamud Yusuf, A;Dzyubenko, E;Kaltwasser, B;Tertel, T;Giebel, B;Gunzer, M;Popa-Wagner, A;Doeppner, TR;Hermann, DM;
Journal of neuroinflammation
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pharmacological Inhibition and Genetic Deletion of Cystathionine Gamma-Lyase in Mice Protects against Organ Injury in Sepsis: A Key Role of Adhesion Molecules on Endothelial Cells
Authors: Manandhar, S;Chambers, S;Miller, A;Ishii, I;Bhatia, M;
International journal of molecular sciences
Species: Transgenic Mouse, Mouse
Sample Types: Whole Tissue
Applications: IHC -
Macrophage-to-endothelial cell crosstalk by the cholesterol metabolite 27HC promotes atherosclerosis in male mice
Authors: Yu, L;Xu, L;Chu, H;Peng, J;Sacharidou, A;Hsieh, HH;Weinstock, A;Khan, S;Ma, L;Durán, JGB;McDonald, J;Nelson, ER;Park, S;McDonnell, DP;Moore, KJ;Huang, LJ;Fisher, EA;Mineo, C;Huang, L;Shaul, PW;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Partial Mural Cell Ablation Disrupts Coronary Vasculature Integrity and Induces Systolic Dysfunction
Authors: Cornuault, L;Hérion, FX;Bourguignon, C;Rouault, P;Foussard, N;Alzieu, P;Chapouly, C;Gadeau, AP;Couffinhal, T;Renault, MA;
Journal of the American Heart Association
Species: Transgenic Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: Immunohistochemistry, Western Blot -
Production of Dermatophagoides farinae Having Low Bacterial Content Using Ampicillin
Authors: Kim, JY;Yi, MH;Kim, M;Choi, JH;Lee, S;Yong, TS;
Journal of immunology research
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
TMEM59 ablation leads to loss of olfactory sensory neurons and impairs olfactory functions via interaction with inflammation
Authors: Z Ma, W Li, L Zhuang, T Wen, P Wang, H Yu, Y Liu, Y Yu
Brain, Behavior, and Immunity, 2023-04-13;111(0):151-168.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lgr5+ cells are required and dynamically participate in olfactory epithelium regeneration: a revisiting shows Lgr5 expression in multiple cell lineages
Authors: W Ren, Z Ma, L Wang, X Feng, H Yu, Y Yu
Theranostics, 2022-07-18;12(13):5631-5644.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Targeted BRD4 protein degradation by dBET1 ameliorates acute ischemic brain injury and improves functional outcomes associated with reduced neuroinflammation and oxidative stress and preservation of blood-brain barrier integrity
Authors: L Liu, C Yang, BP Lavayen, RJ Tishko, J Larochelle, E Candelario
Journal of Neuroinflammation, 2022-06-27;19(1):168.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Novel role for caspase 1 inhibitor VX765 in suppressing NLRP3 inflammasome assembly and atherosclerosis via promoting mitophagy and efferocytosis
Authors: Y Jin, Y Liu, L Xu, J Xu, Y Xiong, Y Peng, K Ding, S Zheng, N Yang, Z Zhang, L Li, L Tan, H Song, J Fu
Cell Death & Disease, 2022-05-31;13(5):512.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pericyte Loss Leads to Capillary Stalling Through Increased Leukocyte-Endothelial Cell Interaction in the Brain
Authors: YG Choe, JH Yoon, J Joo, B Kim, SP Hong, GY Koh, DS Lee, WY Oh, Y Jeong
Frontiers in Cellular Neuroscience, 2022-03-11;16(0):848764.
Species: Mouse
Sample Types: Protein Lysates
Applications: Western Blot -
Critical changes in hypothalamic gene networks in response to pancreatic cancer as found by single-cell RNA sequencing
Authors: C Huisman, MA Norgard, PR Levasseur, SM Krasnow, MGP van der Wi, B Olson, DL Marks
Molecular Metabolism, 2022-01-11;0(0):101441.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Apolipoprotein E receptor 2 deficiency decreases endothelial adhesion of monocytes and protects against autoimmune encephalomyelitis
Authors: Calvier L, Manouchehri N, Sacharidou A et al.
Science Immunology
-
Deficiency of peroxiredoxin 2 exacerbates angiotensin II-induced abdominal aortic aneurysm
Authors: SJ Jeong, MJ Cho, NY Ko, S Kim, IH Jung, JK Min, SH Lee, JG Park, GT Oh
Exp. Mol. Med., 2020-09-14;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
RNA-seq analysis of gene expression profiles in isolated stria vascularis from wild-type and Alport mice reveals key pathways underling Alport strial pathogenesis
Authors: B Dufek, DT Meehan, D Delimont, K Wilhelm, G Samuelson, R Coenen, J Madison, E Doyle, B Smyth, G Phillips, MA Gratton, D Cosgrove
PLoS ONE, 2020-08-21;15(8):e0237907.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Exposure to Zinc Oxide Nanoparticles Disrupts Endothelial Tight and Adherens Junctions and Induces Pulmonary Inflammatory Cell Infiltration
Authors: CM Chen, ML Wu, YC Ho, PY Gung, MH Tsai, AN Orekhov, IA Sobenin, P Lin, SF Yet
Int J Mol Sci, 2020-05-13;21(10):.
Species: Human
Sample Types: Cell Lys
Applications: Western Blot -
A Multivalent ICAM-1 Binding Nanoparticle which Inhibits ICAM-1 and LFA-1 Interaction Represents a New Tool for the Investigation of Autoimmune-Mediated Dry Eye
Authors: PY Hsueh, Y Ju, A Vega, MC Edman, JA MacKay, SF Hamm-Alvar
Int J Mol Sci, 2020-04-15;21(8):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cell-specific and athero-protective roles for RIPK3 in a murine model of atherosclerosis
Authors: S Colijn, V Muthukumar, J Xie, S Gao, CT Griffin
Dis Model Mech, 2020-01-24;13(1):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
IgA1 deposition may induce NLRP3 expression and macrophage transdifferentiation of podocyte in IgA nephropathy
Authors: W Peng, GQ Pei, Y Tang, L Tan, W Qin
J Transl Med, 2019-12-03;17(1):406.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Interleukin 1 alpha administration is neuroprotective and neuro-restorative following experimental ischemic stroke
Authors: KE Salmeron, ME Maniskas, DN Edwards, R Wong, I Rajkovic, A Trout, AA Rahman, S Hamilton, JF Fraser, E Pinteaux, GJ Bix
J Neuroinflammation, 2019-11-14;16(1):222.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VCAM-1 Density and Tumor Perfusion Predict T-cell Infiltration and Treatment Response in Preclinical Models
Authors: J Riegler, H Gill, A Ogasawara, M Hedehus, V Javinal, J Oeh, GZ Ferl, J Marik, S Williams, D Sampath, J Schartner, RAD Carano
Neoplasia, 2019-09-11;21(10):1036-1050.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Intermittent Hypoxia Activates Duration-Dependent Protective and Injurious Mechanisms in Mouse Lung Endothelial Cells
Authors: P Wohlrab, L Soto-Gonza, T Benesch, MP Winter, IM Lang, K Markstalle, V Tretter, KU Klein
Front Physiol, 2018-12-06;9(0):1754.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Early and Late Protective Effect of Bone Marrow Mononuclear Cell Transplantation on Radiation-Induced Vascular Dysfunction and Skin Lesions
Authors: V Holler, V Buard, T Roque, C Squiban, M Benderitte, S Flamant, R Tamarat
Cell Transplant, 2018-11-09;0(0):9636897188103.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Neurokinin-1 receptor antagonism improves postoperative neurocognitive disorder in mice.
Authors: Li Z, Luo T, Ning X, Xiong C, Wu A
Neurosci Lett, 2018-09-28;687(0):189-195.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Complement activation contributes to perioperative neurocognitive disorders in mice
Authors: C Xiong, J Liu, D Lin, J Zhang, N Terrando, A Wu
J Neuroinflammation, 2018-09-04;15(1):254.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance and Neurovascular Adhesion Molecule Expression
Authors: A Niraula, Y Wang, JP Godbout, JF Sheridan
J. Neurosci., 2018-01-30;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Suberanilohydroxamic Acid as a Pharmacological Kruppel-Like Factor 2 Activator That Represses Vascular Inflammation and Atherosclerosis
Authors: Y Xu, S Xu, P Liu, M Koroleva, S Zhang, S Si, ZG Jin
J Am Heart Assoc, 2017-11-30;6(12):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Interferon-? treatment in vitro elicits some of the changes in cathepsin S and antigen presentation characteristic of lacrimal glands and corneas from the NOD mouse model of Sj�gren's Syndrome
Authors: Z Meng, W Klinngam, MC Edman, SF Hamm-Alvar
PLoS ONE, 2017-09-13;12(9):e0184781.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway
Authors: CC Wei, YY Kong, GQ Li, YF Guan, P Wang, CY Miao
Sci Rep, 2017-04-06;7(1):717.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Plasminogen Activator Inhibitor-1 Protects Mice Against Cardiac Fibrosis by Inhibiting Urokinase-type Plasminogen Activator-mediated Plasminogen Activation
Authors: KK Gupta, DL Donahue, MJ Sandoval-C, FJ Castellino, VA Ploplis
Sci Rep, 2017-03-23;7(1):365.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Epidermal RAF prevents allergic skin disease
Elife, 2016-07-19;5(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain
Authors: Caroline M. Sawicki, Daniel B. McKim, Eric S. Wohleb, Brant L. Jarrett, Brenda F. Reader, Diana M. Norden et al.
Neuroscience
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals.
Authors: Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, Olsson A
Cancer Res, 2015-06-12;75(13):2653-62.
-
Platelet-driven leukotriene C4-mediated airway inflammation in mice is aspirin-sensitive and depends on T prostanoid receptors.
Authors: Liu T, Garofalo D, Feng C, Lai J, Katz H, Laidlaw T, Boyce J
J Immunol, 2015-04-22;194(11):5061-8.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Abrogation of plasminogen activator inhibitor-1-vitronectin interaction ameliorates acute kidney injury in murine endotoxemia.
Authors: Gupta K, Donahue D, Sandoval-Cooper M, Castellino F, Ploplis V
PLoS ONE, 2015-03-23;10(3):e0120728.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Low-dose irradiation affects expression of inflammatory markers in the heart of ApoE -/- mice.
Authors: Mathias D, Mitchel R, Barclay M, Wyatt H, Bugden M, Priest N, Whitman S, Scholz M, Hildebrandt G, Kamprad M, Glasow A
PLoS ONE, 2015-03-23;10(3):e0119661.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Intercellular adhesion molecule-1 expression by skeletal muscle cells augments myogenesis.
Authors: Goh Q, Dearth C, Corbett J, Pierre P, Chadee D, Pizza F
Exp Cell Res, 2014-09-30;331(2):292-308.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC-Fr -
ICAM-1-based rabies virus vaccine shows increased infection and activation of primary murine B cells in vitro and enhanced antibody titers in-vivo.
Authors: Norton, James E, Lytle, Andrew G, Shen, Shixue, Tzvetkov, Evgeni P, Dorfmeier, Corin L, McGettigan, James P
PLoS ONE, 2014-01-29;9(1):e87098.
Species: Hamster
Sample Types: Cell Lysates
Applications: Western Blot -
CD44-deficiency attenuates the immunologic responses to LPS and delays the onset of endotoxic shock-induced renal inflammation and dysfunction.
Authors: Rampanelli, Elena, Dessing, Mark C, Claessen, Nike, Teske, Gwendoli, Joosten, Sander P, Pals, Steven T, Leemans, Jaklien, Florquin, Sandrine
PLoS ONE, 2013-12-23;8(12):e84479.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Peptide inhibitor of NF-kappaB translocation ameliorates experimental atherosclerosis.
Authors: Mallavia B, Recio C, Oguiza A, Ortiz-Munoz G, Lazaro I, Lopez-Parra V, Lopez-Franco O, Schindler S, Depping R, Egido J, Gomez-Guerrero C
Am J Pathol, 2013-04-16;182(5):1910-21.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Skeletal muscle cells express ICAM-1 after muscle overload and ICAM-1 contributes to the ensuing hypertrophic response.
Authors: Dearth C, Goh Q, Marino J, Cicinelli P, Torres-Palsa M, Pierre P, Worth R, Pizza F
PLoS ONE, 2013-03-11;8(3):e58486.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells, Whole Tissue
Applications: ICC, IHC, Western Blot -
Biomarkers of disease and treatment in murine and cynomolgus models of chronic asthma.
Authors: Louten J, Mattson JD, Malinao MC, Li Y, Emson C, Vega F, Wardle RL, Van Scott MR, Fick RB, McClanahan TK, de Waal Malefyt R, Beaumont M
Biomark Insights, 2012-07-10;7(0):87-104.
Species: Mouse
Sample Types: BALF
Applications: Western Blot -
Lung-targeted overexpression of the NF-kappaB member RelB inhibits cigarette smoke-induced inflammation.
Authors: McMillan DH, Baglole CJ, Thatcher TH, Maggirwar S, Sime PJ, Phipps RP
Am. J. Pathol., 2011-05-05;179(1):125-33.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Increased expression of CXCR4 and integrin alphaM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency.
Authors: Kubo M, Li TS, Kamota T, Ohshima M, Qin SL, Hamano K
J. Cell. Physiol., 2009-08-01;220(2):508-14.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion.
Authors: Kato N, Yuzawa Y, Kosugi T
J. Am. Soc. Nephrol., 2009-05-14;20(7):1565-76.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-Fr, Western Blot -
Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation.
Authors: Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL, Quertermous T
Am. J. Physiol. Heart Circ. Physiol., 2009-03-20;296(5):H1329-35.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Antigen-loaded ER microsomes from APC induce potent immune responses against viral infection.
Authors: Sofra V, Mansour S, Liu M, Gao B, Primpidou E, Wang P, Li S
Eur. J. Immunol., 2009-01-01;39(1):85-95.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms.
Authors: Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R
Brain Res., 2008-10-09;1244(0):164-72.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Plasminogen activator inhibitor-1 regulates neutrophil influx during acute pyelonephritis.
Authors: Roelofs JJ, Teske GJ, Bonta PI, de Vries CJ, Meijers JC, Weening JJ, van der Poll T, Florquin S
Kidney Int., 2008-09-17;75(1):52-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance.
Authors: Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW
Arterioscler. Thromb. Vasc. Biol., 2008-09-04;28(11):1982-8.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Atherosclerosis prevention by a fish oil-rich diet in apoE(-/-) mice is associated with a reduction of endothelial adhesion molecules.
Authors: Casos K, Saiz MP, Ruiz-Sanz JI, Mitjavila MT
Atherosclerosis, 2008-03-16;201(2):306-17.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein E-deficient and apolipoprotein E/low-density lipoprotein receptor-deficient mice.
Authors: Zhang WJ, Bird KE, McMillen TS, LeBoeuf RC, Hagen TM, Frei B
Circulation, 2007-12-24;117(3):421-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Fractalkine upregulates intercellular adhesion molecule-1 in endothelial cells through CX3CR1 and the Jak Stat5 pathway.
Authors: Yang XP, Mattagajasingh S, Su S, Chen G, Cai Z, Fox-Talbot K, Irani K, Becker LC
Circ. Res., 2007-09-20;101(10):1001-8.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Crosstalk between keratinocytes and adaptive immune cells in an IkappaBalpha protein-mediated inflammatory disease of the skin.
Authors: Rebholz B, Haase I, Eckelt B, Paxian S, Flaig MJ, Ghoreschi K, Nedospasov SA, Mailhammer R, Debey-Pascher S, Schultze JL, Weindl G, Forster I, Huss R, Stratis A, Ruzicka T, Rocken M, Pfeffer K, Schmid RM, Rupec RA
Immunity, 2007-08-09;27(2):296-307.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
High pressure promotes monocyte adhesion to the vascular wall.
Authors: Riou S, Mees B, Esposito B, Merval R, Vilar J, Stengel D, Ninio E, van Haperen R, de Crom R, Tedgui A, Lehoux S
Circ. Res., 2007-03-29;100(8):1226-33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium.
Authors: Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG
J. Exp. Med., 2006-11-20;203(12):2763-77.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Activation of mouse protease-activated receptor-2 induces lymphocyte adhesion and generation of reactive oxygen species.
Authors: Lim SY, Tennant GM, Kennedy S, Wainwright CL, Kane KA
Br. J. Pharmacol., 2006-09-18;149(5):591-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Shedding of soluble ICAM-1 into the alveolar space in murine models of acute lung injury.
Authors: Mendez MP, Morris SB, Wilcoxen S, Greeson E, Moore B, Paine R
Am. J. Physiol. Lung Cell Mol. Physiol., 2005-12-22;290(5):L962-70.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
5-Lipoxygenase Metabolite 4-HDHA Is a Mediator of the Antiangiogenic Effect of omega -3 Polyunsaturated Fatty Acids
Authors: Przemyslaw Sapieha, Andreas Stahl, Jing Chen, Molly R. Seaward, Keirnan L. Willett, Nathan M. Krah et al.
Science Translational Medicine
-
Cell-Type-Specific Interleukin 1 Receptor 1 Signaling in the Brain Regulates Distinct Neuroimmune Activities
Authors: Xiaoyu Liu, Daniel P. Nemeth, Daniel B. McKim, Ling Zhu, Damon J. DiSabato, Olimpia Berdysz et al.
Immunity
-
Microglia alter the threshold of spreading depolarization and related potassium uptake in the mouse brain
Authors: Dániel P Varga, Ákos Menyhárt, Balázs Pósfai, Eszter Császár, Nikolett Lénárt, Csaba Cserép et al.
Journal of Cerebral Blood Flow & Metabolism
-
Homozygous Smpd1 deficiency aggravates brain ischemia/ reperfusion injury by mechanisms involving polymorphonuclear neutrophils, whereas heterozygous Smpd1 deficiency protects against mild focal cerebral ischemia
Authors: Nina Hagemann, Ayan Mohamud Yusuf, Carlotta Martiny, Xiaoni Zhang, Christoph Kleinschnitz, Matthias Gunzer et al.
Basic Research in Cardiology
-
Interleukin-1 mediates ischaemic brain injury via distinct actions on endothelial cells and cholinergic neurons.
Authors: Wong R, Lenart N, Hill L et al.
Flow Cytometry Basics for the Non-Expert.
-
Circulating soluble endoglin modifies the inflammatory response in mice
Authors: L Ruiz-Remol, C Ollauri-Ib, L Pérez-Roqu, E Núñez-Góme, F Pérez-Barr, JM López-Novo, M Pericacho, A Rodríguez-
PLoS ONE, 2017-11-16;12(11):e0188204.
-
Re-purposing the pro-senescence properties of doxorubicin to introduce immunotherapy in breast cancer brain metastasis
Authors: Rebeca Uceda-Castro, Andreia S. Margarido, Lesley Cornet, Serena Vegna, Kerstin Hahn, Ji-Ying Song et al.
Cell Reports Medicine
-
Helper-Dependent Adenovirus Transduces the Human and Rat Retina but Elicits an Inflammatory Reaction When Delivered Subretinally in Rats
Authors: Ian C. Han, Erin R. Burnight, Mallory J. Ulferts, Kristan S. Worthington, Stephen R. Russell, Elliott H. Sohn et al.
Human Gene Therapy
-
Expression of intercellular adhesion molecule-1 by myofibers in mdx mice
Authors: Maria J. Torres-Palsa, Matthew V. Koziol, Qingnian Goh, Peter A. Cicinelli, Jennifer M. Peterson, Francis X. Pizza
Muscle & Nerve
-
Neutrophil extracellular traps drive inflammatory pathogenesis in malaria.
Authors: Knackstedt S L, Georgiadou A et al.
Sci Immunol
-
Effects of intra-abdominal sepsis on atherosclerosis in mice
Authors: Ata Murat Kaynar, Sachin Yende, Lin Zhu, Daniel R Frederick, Robin Chambers, Christine L Burton et al.
Critical Care
-
Characterization of Influenza A Virus Infection in Mouse Pulmonary Stem/Progenitor Cells
Authors: Tai-Ling Chao, Sing-Yi Gu, Pi-Han Lin, Yu-Tien Chou, Thai-Yen Ling, Sui-Yuan Chang
Frontiers in Microbiology
-
Age-associated pro-inflammatory adaptations of the mouse thoracic aorta
Authors: Bianca Hemmeryckx, Marc F. Hoylaerts, Eveline Deloose, Cor E. Van Hove, Paul Fransen, Hidde Bult et al.
Thrombosis and Haemostasis
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsIsotype Controls
Reconstitution Buffers
Secondary Antibodies
Reviews for Mouse ICAM-1/CD54 Antibody
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Mouse ICAM-1/CD54 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
paraffin-embedded tissue
Antigen Retrieval in citrate buffer pH 6
concentrations tested: 1:50-1:300 all worked