Mouse LRIG1 Antibody Summary
Ala37-Thr794
Accession # P70193
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Mouse LRIG1 by Western Blot. Western blot shows lysates of bEnd.3 mouse endothelioma cell line untreated (-), treated (+) or mock treated (m) with 30 pmol mouse LRIG1 or mouse ERK1 siRNA. PVDF membrane was probed with 2 µg/mL of Goat Anti-Mouse LRIG1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3688) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). Specific bands were detected for LRIG1 at approximately 140 kDa and 70 kDa (as indicated, upper panel). For additional reference ERK1 was detected using Rabbit Anti-Human/Mouse/Rat ERK1 Antigen Affinity-purified Polyclonal Antibody (lower panel, Catalog # AF1575) This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of LRIG1 in bEnd.3 Mouse Cell Line by Flow Cytometry. bEnd.3 mouse endothelioma cell line was stained with Goat Anti-Mouse LRIG1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3688, filled histogram) or control antibody (Catalog # AB-108-C, open histogram), followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0107).
 View Larger
View Larger
LRIG1 in bEnd.3 Mouse Cell Line. LRIG1 was detected in immersion fixed bEnd.3 mouse endothelioma cell line using Goat Anti-Mouse LRIG1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3688) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red, upper panel; Catalog # NL001) and counterstained with DAPI (blue, lower panel). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Detection of Mouse LRIG1 by Immunohistochemistry JunB expression during skin maturation and upon stress. a Representative microphotographs with double immunostaining for JunB (green) and FABP5 (red), indicative of sebaceous glands, in skin derived from 1, 3, and 5 days old mice. Inset showing magnified view of a sebaceous gland. Scale bars, 50 µm. b Immunostaining of JunB (green) and FABP5 (red) in 60 days old mice. Inset showing magnified view. c Representative microphotographs with double immunostaining of JunB (green) and FABP5 (red) in dorsal skin of 60 days old mice following hair plucking or d after topical TPA application, a potent inducer of proliferation. e Immunostaining of JunB (green) and LRIG1 or CD34 (red) in dorsal skin of 60 days old mice following hair plucking. f Immunostaining of JunB (green) and FABP5 (red) in adult human skin. E, epidermis; D, dermis; HF, hair follicle; SG, sebaceous gland; SD, sebaceous duct; HS, hair shaft. Asterisk indicates hair shaft autofluorescence. Dashed line indicates the epidermal-dermal junction. Scale bars, 50 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30143626), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse LRIG1 by Immunohistochemistry Lrigs are co-expressed in the embryonic inner ear.(A) Diagram of the immature inner ear structure at E12.5 (left) and the mature structure at E16 (right), with schematic cross sections cut transverse to the ear at each age shown below. For the E16 cross section, the sensory epithelia are labeled red and the neurons green. In situs for Lrig1 (B) and X-gal staining for Lrig3-beta geo activity (C) show overlapping expression for Lrig1 and Lrig3 in the atrium (arrowhead) and the non-sensory domain of the cochlea. On the other hand, Lrig2-beta geo is active throughout the developing otic epithelium (D). c = cochlea, ed = endolymphatic duct, lp = lateral pouch, vp = vertical pouch. Scale bar = 50 µm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24086156), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse LRIG1 by Immunocytochemistry/Immunofluorescence Lrig1 and Lrig2-beta geo are co-expressed in non-sensory tissues and in the vestibular ganglion.Transverse sections through Lrig2+/− tissue at E12.5 (A–C), E16.5 (D–G), and P15 (H–K) were double labeled with combinations of antibodies to Lrig1, beta -galactosidase (to detect Lrig2-beta geo), Sox2, and neurofilament (NF). (A) At E12.5, Lrig1 was detected in the atrium (bracket), while Lrig2-beta geo was present throughout the otic epithelium and surrounding mesenchyme (A″), overlapping with Lrig1 in the atrium (B). Within the atrium, Lrig1 was present in non-sensory tissues that flank Sox-2 positive sensory regions (arrowheads, C–C″). This expression was maintained at E16.5, with Lrig1 in the transitional epithelium adjacent to the utricular macula (arrowhead, D′) and in the extramacular epithelium of the saccule (arrowhead, F′), as well as in vestibular projections to the utricle and lateral crista (arrow, D′). Lrig2-beta geo, on the other hand, continued to be expressed broadly in both sensory and non-sensory portions of the vestibular organs at E16.5 (E′, G′). After the onset of hearing (P15), Lrig1 was expressed in NF-positive fibers innervating the utricular and saccular maculae (arrows, I, J), whereas Lrig2-beta geo was enriched in all vestibular sensory epithelia (I′,J′,K), which were recognized by the presence of NF labeled projections. c = crista, cd = cochlear duct, ed = endolymphatic duct, hb = hindbrain, lp = lateral pouch, sg = spiral ganglion, sm = saccular macula, um = utricular macule, vg = vestibular ganglion, vp = vertical pouch, VIIIV = vestibular division of the eighth cranial nerve. Scale bar = 40 µm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24086156), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse LRIG1 by Immunocytochemistry/Immunofluorescence Lrig1 and Lrig2-beta geo are co-expressed in the non-sensory region of the cochlea.Transverse sections through Lrig2+/− tissue at E12.5 (A), E16.5 (B), and P15 (C) were double labeled with antibodies to Lrig1, beta -galactosidase, and NF. (A) At E12.5, staining was evident in the non-sensory region of the cochlear epithelium (asterisk) and the mesenchyme surrounding the spiral ganglion. (B) At E16.5, Lrig1 was detected in the medial wall of the cochlea, which will form the inner sulcus and Reissner's membrane (asterisk). (C) At P15, Lrig1 was found in the base of Reissner's membrane (asterisk), with localization to the cell surface (inset). In contrast, at E12.5 and 16.5, Lrig2-beta geo was found broadly in the cochlear epithelium and surrounding mesenchyme (A′–B′). At P15, expression was enriched in spiral ganglion neurons and in the organ of Corti (C′). cd = cochlear duct, m = mesenchyme, oC = organ of Corti, rm = Reissner's membrane, sg = spiral ganglion. Scale bar = 40 µm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24086156), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse LRIG1 by Immunocytochemistry/Immunofluorescence Association of Oct1 with normal somatic stem cells.A. IF images of cross-sections from grossly uninvolved colon margins of a male familial adenomatous polyposis patient. Frozen sections were stained with mouse anti-Oct1 antibodies (Millipore MAB5434) and co-stained with TO-PRO. Crypts are shown in cross-section. White dashed circle highlights a crypt. Arrows indicate cells staining strongly for Oct1. B. IF images of colon crypt sections from a normal male individual. Sections were stained with DAPI, and anti-Oct1 and anti-ALDH1a1 antibodies. Merged images are shown at right. White dashed lines highlight crypts. Examples of cells co-staining with Oct1 and ALDH1a1 are highlighted with yellow arrows. An example cell staining with ALDH1a1 only is highlighted with an asterisk. Inset at lower right-hand corner is a digital magnification of the central portion of the image. Sections were formalin-fixed and paraffin-embedded. C. Frozen mouse colon tissue sections were stained with DAPI, and anti-Lrig1 and anti-Oct1 antibodies. IF images of longitudinal sections are shown. White dashed lines highlight the crypt. D. IF images of mouse small intestine sections. Sections were stained with DAPI and anti-Oct1 antibodies. Merged images are shown at right. White dashed lines highlight a crypt. Inset at upper right-hand corner is a digital magnification of the central portion of the image. Sections were formalin-fixed and paraffin-embedded. E. IF images of cross-sectional duodenum sections from a normal C57BL/6 mouse. Frozen sections were stained with DAPI and anti-Oct1 and anti-Lgr5 antibodies. Merged images are shown at right. Examples of co-staining cells are highlighted with yellow arrows. White dashed lines highlights crypts. F. Longitudinal sections. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23144633), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
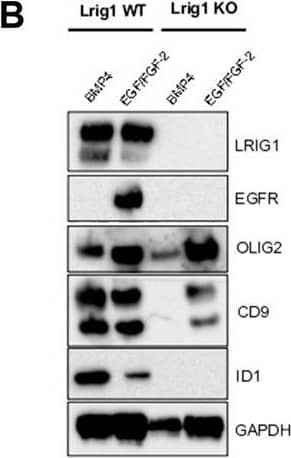
Detection of Mouse LRIG1 by Western Blot Lrig1 KO GSCs show impaired BMP signalling (A) Phase-contrast imaging of Lrig1 WT and Lrig1 KO GSCs treated with EGF/FGF or BMP4 for 3 days. (B) Immunoblot for LRIG1, EGFR, OLIG2, CD9, ID1 and GAPDH expression in Lrig1 WT and Lrig1 KO GSCs treated with EGF/FGF or BMP4 for 3 days. (C) Immunoblot and quantification for PCNA and GAPDH in Lrig1 WT and Lrig1 KO GSCs grown in EGF/FGF-2 or treated with 5 ng/ml or 10 ng/ml of BMP4 for 3 days. Quantification shown relative to GAPDH and Lrig1 WT EGF/FGF-2 control. (D) Immunoblot and quantification for MCM2 and GAPDH in Lrig1 WT and Lrig1 KO GSCs grown in EGF/FGF-2 or treated with 5 ng/ml or 10 ng/ml of BMP4 for 3 days. Quantification shown relative to GAPDH and Lrig1 WT EGF/FGF-2 control. (E) Immunoblot for LRIG1, pSMAD1/5, ID1, BLBP, pERK1/2 and GAPDH in Lrig1 WT and Lrig1 KO GSCs treated with different dosages of BMP4 for 3 days. Dosages in ng/ml. Protein band sizes shown in kDa. (F) Flow cytometry plots showing strategy to select Lrig1-positive cells in Lrig1 KO GSCs after reintroduction of mLrig1-FLAG. (G) Immunostaining for pSMAD1/5 (red) and nuclear counterstaining with DAPI (blue). +Lrig1-FLAG refer to the sorted populations. ICC was performed on Fucci PE Lrig1 WT, Fucci PE Lrig1 KO and the populations sorted for Lrig1-FLAG following expansion. (H) Quantification of the percentage of pSMAD 1/5 in the different conditions (n = 3) Unpaired two-tailed t-tests. Scale bar in (A) is 50 μm and (H) is 20 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36420140), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
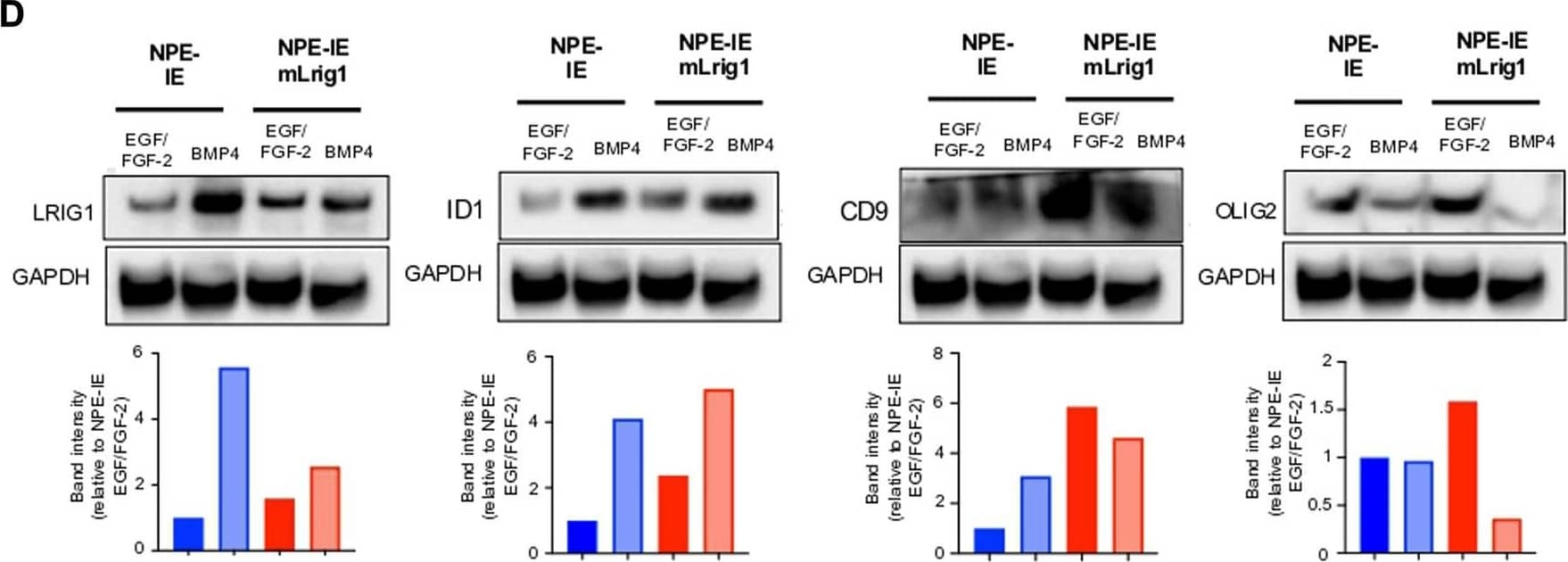
Detection of Mouse LRIG1 by Western Blot Overexpression of Lrig1 reduces GSC proliferation. (A) Representative fluorescence imaging of DAPI stained colonies using Operetta high-content analysis system for NPE-IE control and NPE-IE mLrig1 overexpressing cells. (B) Quantification of the number of colonies formed in single cell colony forming assays by NPE-IE control and NPE-IE mLrig1 overexpressing cells (n = 3) (C) Quantification of the size of colonies formed in single cell colony forming assays by NPE-IE control and NPE-IE mLrig1 overexpressing cells (n = 3) p = 0.0456. Each dot represents a single colony. (D) Immunoblot and quantification of LRIG1, OLIG2, ID1, CD9 and GAPDH (loading control) in NPE-IE control and NPE-IE mLrig1 overexpressing GSCs treated with EGF/FGF or BMP4 for 3 days. Quantification shown relative to GAPDH and NPE-IE EGF/FGF-2 control. Scale bar in (A) is 500 μm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36420140), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
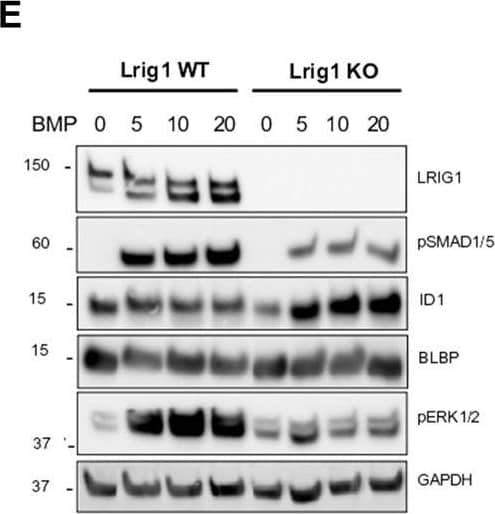
Detection of Mouse LRIG1 by Western Blot Lrig1 KO GSCs show impaired BMP signalling (A) Phase-contrast imaging of Lrig1 WT and Lrig1 KO GSCs treated with EGF/FGF or BMP4 for 3 days. (B) Immunoblot for LRIG1, EGFR, OLIG2, CD9, ID1 and GAPDH expression in Lrig1 WT and Lrig1 KO GSCs treated with EGF/FGF or BMP4 for 3 days. (C) Immunoblot and quantification for PCNA and GAPDH in Lrig1 WT and Lrig1 KO GSCs grown in EGF/FGF-2 or treated with 5 ng/ml or 10 ng/ml of BMP4 for 3 days. Quantification shown relative to GAPDH and Lrig1 WT EGF/FGF-2 control. (D) Immunoblot and quantification for MCM2 and GAPDH in Lrig1 WT and Lrig1 KO GSCs grown in EGF/FGF-2 or treated with 5 ng/ml or 10 ng/ml of BMP4 for 3 days. Quantification shown relative to GAPDH and Lrig1 WT EGF/FGF-2 control. (E) Immunoblot for LRIG1, pSMAD1/5, ID1, BLBP, pERK1/2 and GAPDH in Lrig1 WT and Lrig1 KO GSCs treated with different dosages of BMP4 for 3 days. Dosages in ng/ml. Protein band sizes shown in kDa. (F) Flow cytometry plots showing strategy to select Lrig1-positive cells in Lrig1 KO GSCs after reintroduction of mLrig1-FLAG. (G) Immunostaining for pSMAD1/5 (red) and nuclear counterstaining with DAPI (blue). +Lrig1-FLAG refer to the sorted populations. ICC was performed on Fucci PE Lrig1 WT, Fucci PE Lrig1 KO and the populations sorted for Lrig1-FLAG following expansion. (H) Quantification of the percentage of pSMAD 1/5 in the different conditions (n = 3) Unpaired two-tailed t-tests. Scale bar in (A) is 50 μm and (H) is 20 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36420140), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
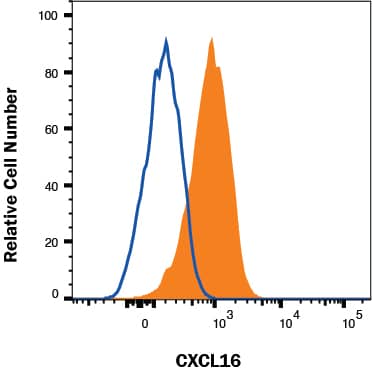
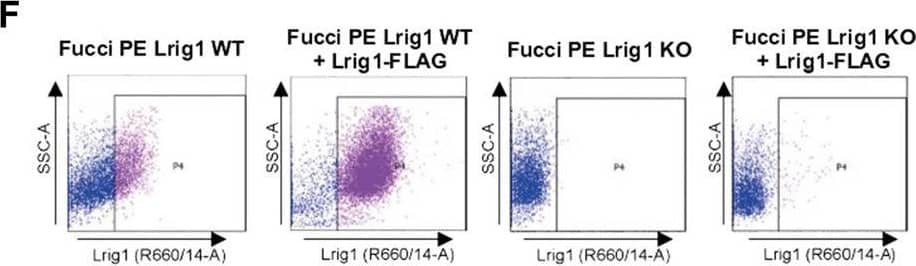
Detection of Mouse LRIG1 by Flow Cytometry Lrig1 KO GSCs show impaired BMP signalling (A) Phase-contrast imaging of Lrig1 WT and Lrig1 KO GSCs treated with EGF/FGF or BMP4 for 3 days. (B) Immunoblot for LRIG1, EGFR, OLIG2, CD9, ID1 and GAPDH expression in Lrig1 WT and Lrig1 KO GSCs treated with EGF/FGF or BMP4 for 3 days. (C) Immunoblot and quantification for PCNA and GAPDH in Lrig1 WT and Lrig1 KO GSCs grown in EGF/FGF-2 or treated with 5 ng/ml or 10 ng/ml of BMP4 for 3 days. Quantification shown relative to GAPDH and Lrig1 WT EGF/FGF-2 control. (D) Immunoblot and quantification for MCM2 and GAPDH in Lrig1 WT and Lrig1 KO GSCs grown in EGF/FGF-2 or treated with 5 ng/ml or 10 ng/ml of BMP4 for 3 days. Quantification shown relative to GAPDH and Lrig1 WT EGF/FGF-2 control. (E) Immunoblot for LRIG1, pSMAD1/5, ID1, BLBP, pERK1/2 and GAPDH in Lrig1 WT and Lrig1 KO GSCs treated with different dosages of BMP4 for 3 days. Dosages in ng/ml. Protein band sizes shown in kDa. (F) Flow cytometry plots showing strategy to select Lrig1-positive cells in Lrig1 KO GSCs after reintroduction of mLrig1-FLAG. (G) Immunostaining for pSMAD1/5 (red) and nuclear counterstaining with DAPI (blue). +Lrig1-FLAG refer to the sorted populations. ICC was performed on Fucci PE Lrig1 WT, Fucci PE Lrig1 KO and the populations sorted for Lrig1-FLAG following expansion. (H) Quantification of the percentage of pSMAD 1/5 in the different conditions (n = 3) Unpaired two-tailed t-tests. Scale bar in (A) is 50 μm and (H) is 20 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36420140), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
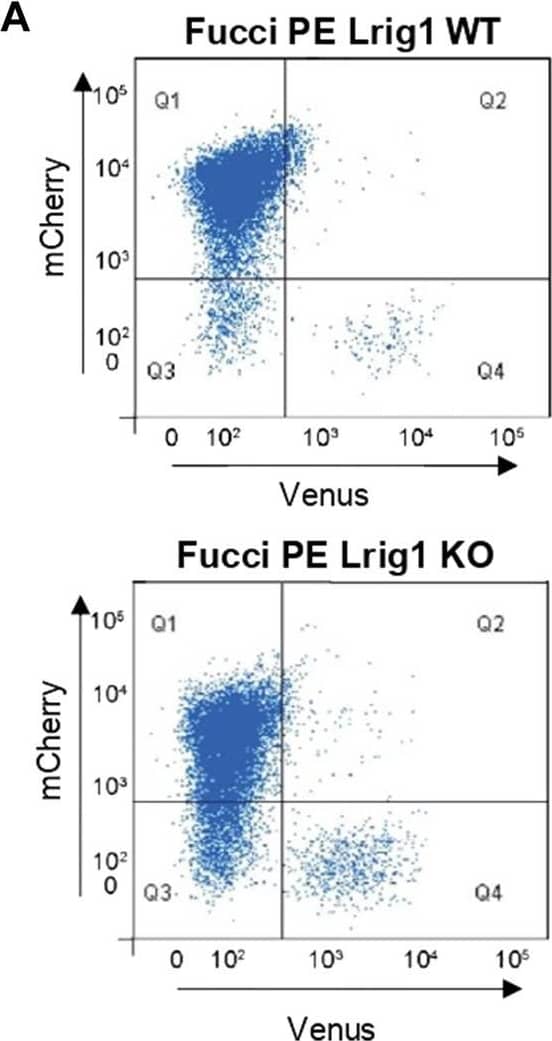
Detection of Mouse LRIG1 by Flow Cytometry Lrig1 regulates proliferation of GSCs. (A) Flow analysis of Fucci mCherry-Cdt1 and aVenus-hGem reporters in Lrig1 WT and Lrig1 KO mouse GSCs. (n = 3). (B) Quantification of the number of colonies formed in single cell colony forming assays by Fucci NSCs, Fucci PE Lrig1 WT (GSCs with p53 KO and EGFRvIII overexpression) and Fucci PE Lrig1 KO. n = 3 (mean ± SD). (C) Quantification of the size of colonies formed in single cell colony forming assays by Fucci NSC, Fucci PE Lrig1 WT (GSCs with p53 KO and EGFRvIII overexpression) and Fucci PE Lrig1 KO as assessed by DAPI (n = 3 independent experiments, median ± SD, each dot represents a single colony). p value = 0.0456. (D) Representative fluorescence imaging of DAPI stained colonies using Operetta high-content analysis system. Scale bar in (D) is 1,500 μm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36420140), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: LRIG1
LRIG-1 (leucine-rich repeats and Ig-like domains-1; also LIG-1) is an approximately 130-145 kDa glycoprotein that belongs to the LRIG gene family. It is widely expressed, and appears on the surface of prostatic epithelium, endothelial cells, vascular and visceral smooth muscle, mammary epithelium, cardiac muscle, keratinocytes and neurons. LRIG-1 is believed to negatively regulate the ErbB family of receptors. In particular, and in a ligand-independent manner, LRIG-1 complexes with all four ErbBs, promoting their ubiquitination and decreasing their number. Alternatively, LRIG-1 is suggested to bind to the ErbBs, preventing their dimerization and signal transduction. Mature mouse LRIG-1 is a 1057 amino acid (aa) type I transmembrane protein (SwissProt #:P70193). It contains a large 762 amino acid (aa) extracellular domain (ECD) (aa 35-795) plus a 274 aa cytoplasmic region. The ECD contains 17 LRRs (aa’s 35-491) and three C2-type Ig-like domains (aa’s 497-781). These two domain types are each sufficient for EGFR binding. There one potential alternative splice form that a deletion of aa 875-923. The LRIG-1 ECD undergoes proteolysis, generating 90-105 and 60-70 kDa soluble fragments. Over aa 37-794, human LRIG-1 shares 97% and 90% aa sequence identity with rat and human LRIG-1, respectively.
Product Datasheets
Citations for Mouse LRIG1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
35
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
In Vivo Analysis of Lrig Genes Reveals Redundant and Independent Functions in the Inner Ear
Authors: Tony del Rio, Allison M. Nishitani, Wei-Ming Yu, Lisa V. Goodrich
PLoS Genetics
-
WNT10A mutation causes ectodermal dysplasia by impairing progenitor cell proliferation and KLF4-mediated differentiation
Authors: M Xu, J Horrell, M Snitow, J Cui, H Gochnauer, CM Syrett, S Kallish, JT Seykora, F Liu, D Gaillard, JP Katz, KH Kaestner, B Levin, C Mansfield, JE Douglas, BJ Cowart, M Tordoff, F Liu, X Zhu, LA Barlow, AI Rubin, JA McGrath, EE Morrisey, EY Chu, SE Millar
Nat Commun, 2017-06-07;8(0):15397.
-
Mutant Lef1 controls Gata6 in sebaceous gland development and cancer
Authors: Bénédicte Oulès, Emanuel Rognoni, Esther Hoste, Georgina Goss, Ryan Fiehler, Ken Natsuga et al.
The EMBO Journal
-
Basal Cell Carcinoma Preferentially Arises from Stem Cells within Hair Follicle and Mechanosensory Niches
Authors: Shelby C. Peterson, Markus Eberl, Alicia N. Vagnozzi, Abdelmadjid Belkadi, Natalia A. Veniaminova, Monique E. Verhaegen et al.
Cell Stem Cell
-
Intersections between Regulated Cell Death and Autophagy
Authors: Francesco Napoletano, Olga Baron, Peter Vandenabeele, Bertrand Mollereau, Manolis Fanto
Trends in Cell Biology
-
Urban Particulate Matter Triggers Meibomian Gland Dysfunction
Authors: Tu, M;Liu, R;Xue, J;Xiao, B;Li, J;Liang, L;
Investigative ophthalmology & visual science
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Tracheal separation is driven by NKX2-1-mediated repression of Efnb2 and regulation of endodermal cell sorting
Authors: AE Lewis, A Kuwahara, J Franzosi, JO Bush
Cell Reports, 2022-03-15;38(11):110510.
Species: Mouse
Sample Types: Whole Tissue
Applications: IF -
Cutaneous Effects of In Utero and Lactational Exposure of C57BL/6J Mice to 2,3,7,8-Tetrachlorodibenzo-p-dioxin
Authors: J Bhuju, KM Olesen, CS Muenyi, TS Patel, RW Read, L Thompson, O Skalli, Q Zheng, EA Grice, CH Sutter, TR Sutter
Toxics, 2021-08-20;9(8):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lgr6 marks epidermal stem cells with a nerve-dependent role in wound re-epithelialization
Authors: S Huang, P Kuri, Y Aubert, M Brewster, N Li, O Farrelly, G Rice, H Bae, S Prouty, T Dentchev, W Luo, BC Capell, P Rompolas
Cell Stem Cell, 2021-06-07;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
LRIG1-Mediated Inhibition of EGF Receptor Signaling Regulates Neural Precursor Cell Proliferation in the Neocortex
Authors: D Jeong, D Lozano Cas, A Gengathara, K Edwards, A Saghatelya, DR Kaplan, FD Miller, SA Yuzwa
Cell Rep, 2020-10-13;33(2):108257.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Delineating the early transcriptional specification of the mammalian trachea and esophagus
Authors: A Kuwahara, AE Lewis, C Coombes, FS Leung, M Percharde, JO Bush
Elife, 2020-06-09;9(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A single-progenitor model as the unifying paradigm of epidermal and esophageal epithelial maintenance in mice
Authors: G Piedrafita, V Kostiou, A Wabik, B Colom, D Fernandez-, A Herms, K Murai, BA Hall, PH Jones
Nat Commun, 2020-03-18;11(1):1429.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The aging skin microenvironment dictates stem cell behavior
Authors: Y Ge, Y Miao, S Gur-Cohen, N Gomez, H Yang, M Nikolova, L Polak, Y Hu, A Verma, O Elemento, JG Krueger, E Fuchs
Proc. Natl. Acad. Sci. U.S.A., 2020-02-24;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Epidermal Tissue Adapts to Restrain Progenitors Carrying Clonal p53 Mutations
Authors: K Murai, G Skrupskely, G Piedrafita, M Hall, V Kostiou, SH Ong, T Nagy, A Cagan, D Goulding, AM Klein, BA Hall, PH Jones
Cell Stem Cell, 2018-09-27;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
JunB defines functional and structural integrity of the epidermo-pilosebaceous unit in the skin
Authors: K Singh, E Camera, L Krug, A Basu, RK Pandey, S Munir, M Wlaschek, S Kochanek, M Schorpp-Ki, M Picardo, P Angel, C Niemann, P Maity, K Scharffett
Nat Commun, 2018-08-24;9(1):3425.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration
Authors: S Yui, L Azzolin, M Maimets, MT Pedersen, RP Fordham, SL Hansen, HL Larsen, J Guiu, MRP Alves, CF Rundsten, JV Johansen, Y Li, CD Madsen, T Nakamura, M Watanabe, OH Nielsen, PJ Schweiger, S Piccolo, KB Jensen
Cell Stem Cell, 2017-12-14;22(1):35-49.e7.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Secretory phospholipase A2-IIA overexpressing mice exhibit cyclic alopecia mediated through aberrant hair shaft differentiation and impaired wound healing response
Authors: GL Chovatiya, RM Sarate, RR Sunkara, NP Gawas, V Kala, SK Waghmare
Sci Rep, 2017-09-14;7(1):11619.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Stem cell plasticity enables hair regeneration following Lgr5(+) cell loss
Authors: JD Hoeck, B Biehs, AV Kurtova, NM Kljavin, F de Sousa E, B Alicke, H Koeppen, Z Modrusan, R Piskol, FJ de Sauvage
Nat. Cell Biol., 2017-05-29;19(6):666-676.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Stem Cell Lineage Infidelity Drives Wound Repair and Cancer
Authors: Y Ge, NC Gomez, RC Adam, M Nikolova, H Yang, A Verma, CP Lu, L Polak, S Yuan, O Elemento, E Fuchs
Cell, 2017-04-20;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Interfering with stem cell-specific gatekeeper functions controls tumour initiation and malignant progression of skin tumours.
Authors: Petersson, Monika, Reuter, Karen, Brylka, Heike, Kraus, Andreas, Schettina, Peter, Niemann, Catherin
Nat Commun, 2015-01-22;6(0):5874.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
GPR39 marks specific cells within the sebaceous gland and contributes to skin wound healing.
Authors: Zhao, Huashan, Qiao, Jingqiao, Zhang, Shoubing, Zhang, Huishan, Lei, Xiaohua, Wang, Xinyue, Deng, Zhili, Ning, Lina, Cao, Yujing, Guo, Yong, Liu, Shuang, Duan, Enkui
Sci Rep, 2015-01-21;5(0):7913.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Overexpression of epigen during embryonic development induces reversible, epidermal growth factor receptor-dependent sebaceous gland hyperplasia.
Authors: Dahlhoff M, Frances D, Kloepper J, Paus R, Schafer M, Niemann C, Schneider M
Mol Cell Biol, 2014-06-02;34(16):3086-95.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Kindlin-1 controls Wnt and TGF-beta availability to regulate cutaneous stem cell proliferation.
Authors: Rognoni E, Widmaier M, Jakobson M, Ruppert R, Ussar S, Katsougkri D, Bottcher R, Lai-Cheong J, Rifkin D, McGrath J, Fassler R
Nat Med, 2014-03-30;20(4):350-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC, IHC-P -
Distinct fibroblast lineages determine dermal architecture in skin development and repair.
Authors: Driskell R, Lichtenberger B, Hoste E, Kretzschmar K, Simons B, Charalambous M, Ferron S, Herault Y, Pavlovic G, Ferguson-Smith A, Watt F
Nature, 2013-12-12;504(7479):277-81.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Transcription factor Oct1 is a somatic and cancer stem cell determinant.
Authors: Maddox, Jessica, Shakya, Arvind, South, Samuel, Shelton, Dawne, Andersen, Jared N, Chidester, Stephani, Kang, Jinsuk, Gligorich, Keith M, Jones, David A, Spangrude, Gerald J, Welm, Bryan E, Tantin, Dean
PLoS Genet, 2012-11-08;8(11):e1003048.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling.
Authors: Wong, Vivian W, Stange, Daniel E, Page, Mahalia, Buczacki, Simon, Wabik, Agnieszk, Itami, Satoshi, van de Wetering, Marc, Poulsom, Richard, Wright, Nicholas, Trotter, Matthew, Watt, Fiona M, Winton, Doug J, Clevers, Hans, Jensen, Kim B
Nat Cell Biol, 2012-03-04;14(4):401-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Delayed cutaneous wound healing and aberrant expression of hair follicle stem cell markers in mice selectively lacking Ctip2 in epidermis.
Authors: Liang X, Bhattacharya S, Bajaj G
PLoS ONE, 2012-02-21;7(2):e29999.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis.
Authors: Petersson M, Brylka H, Kraus A
EMBO J., 2011-06-21;30(15):3004-18.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis.
Authors: Jensen KB, Driskell RR, Watt FM
Nat Protoc, 2010-04-22;5(5):898-911.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation.
Authors: Baker CM, Verstuyf A, Jensen KB
Dev. Biol., 2010-04-14;343(1):40-50.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Astrocyte-neuron crosstalk through Hedgehog signaling mediates cortical synapse development
Authors: Y Xie, AT Kuan, W Wang, ZT Herbert, O Mosto, O Olukoya, M Adam, S Vu, M Kim, D Tran, N Gómez, C Charpentie, I Sorour, TE Lacey, MY Tolstoruko, BL Sabatini, WA Lee, CC Harwell
Cell Reports, 2022-02-22;38(8):110416.
-
Niche-Specific Factors Dynamically Regulate Sebaceous Gland Stem Cells in the Skin
Authors: Natalia A. Veniaminova, Marina Grachtchouk, Owen J. Doane, Jamie K. Peterson, David A. Quigley, Madison V. Lull et al.
Developmental Cell
-
Using a new Lrig1 reporter mouse to assess differences between two Lrig1 antibodies in the intestine
Authors: Emily J. Poulin, Anne E. Powell, Yang Wang, Yina Li, Jeffrey L. Franklin, Robert J. Coffey
Stem Cell Research
-
Dermal EZH2 orchestrates dermal differentiation and epidermal proliferation during murine skin development
Authors: Venkata Thulabandu, Timothy Nehila, James W. Ferguson, Radhika P. Atit
Developmental Biology
-
Differential toxicity to murine small and large intestinal epithelium induced by oncology drugs
Authors: Jake M. Bieber, Laura E. Sanman, Xiaoxiao Sun, Heinz Hammerlindl, Feng Bao, Maike A. Roth et al.
Communications Biology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsIsotype Controls
Reconstitution Buffers
Secondary Antibodies
Reviews for Mouse LRIG1 Antibody
There are currently no reviews for this product. Be the first to review Mouse LRIG1 Antibody and earn rewards!
Have you used Mouse LRIG1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image