Th2 Cell Markers
Click on one of the helper T cell subsets shown in the buttons below to see the markers that are most commonly used to identify that cell type.
Recommended Products
Overview
T helper type 2 (Th2) cells are a subset of CD4+ effector T cells that is required for humoral immunity and is important for host defense against large extracellular pathogens such as helminths. Following activation, naïve CD4+ T cells differentiate into Th2 cells in the presence of IL-4 and either IL-2, IL-7, or thymic stromal lymphopoietin (TSLP). Th2 cells are commonly distinguished from other CD3+CD4+CD8- T helper cell subsets based on expression of the cell surface markers CCR3, CCR4, CCR8, CXCR4, and ST2/IL-1 R4, along with the transcriptional regulators, STAT5, STAT6, and GATA-3, the last of which is the master transcriptional regulator required for Th2 cell development. In addition to these markers, the cytokines commonly secreted by Th2 cells, including IL-4, IL-5, IL-9, IL-13, and IL-17E/IL-25 can also be used to distinguish Th2 cells from other CD4+ effector T cell subsets. Th2-associated cytokines promote the survival and proliferation of mast cells, induce chemotaxis of mast cells, basophils, and eosinophils, regulate the production of IgE antibodies by B cells, and promote alternative macrophage activation. Unregulated Th2 responses have been associated with the development of atopic diseases such as allergic rhinitis and asthma.
Data Examples
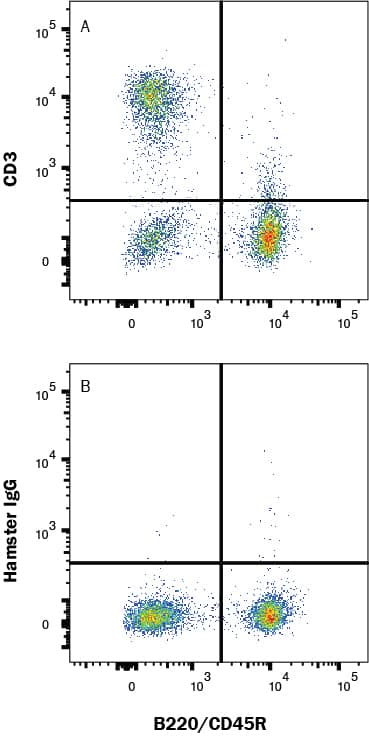
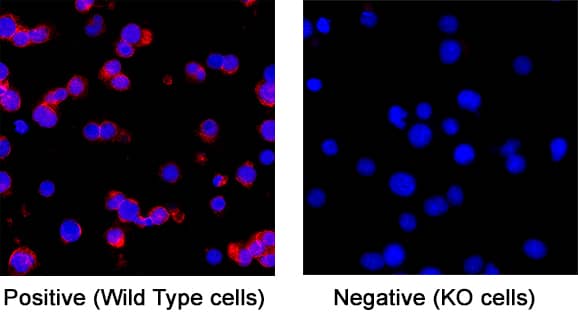
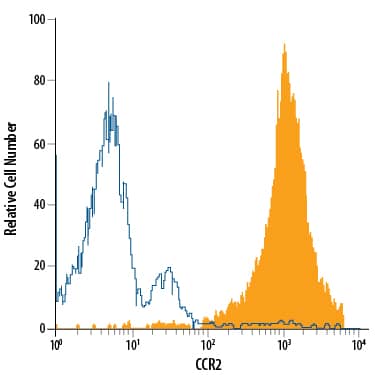
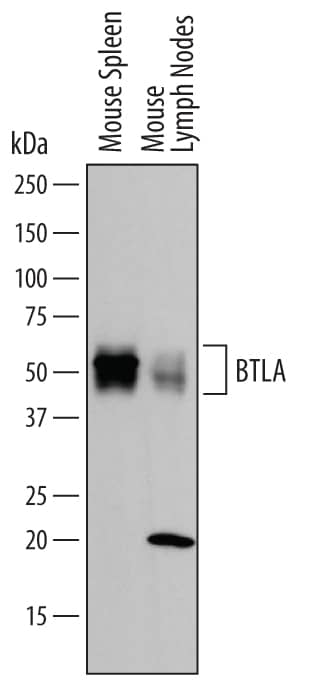
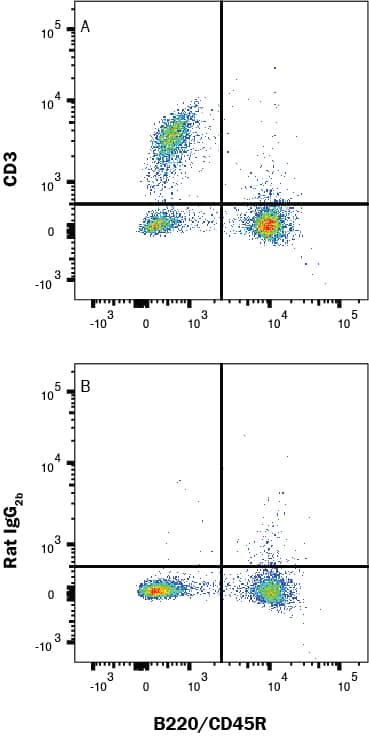
Detection of Cell Surface and Intracellular Markers on Human Th2 Cells by Flow Cytometry. CD4+ T cells were isolated from total human peripheral blood mononuclear cells using a cell selection protocol, such as the one found in the MagCellect™ Human CD4+ T Cell Isolation Kit (R&D Systems, Catalog # MAGH102). Isolated cells were incubated at 37 ˚C for five days in media containing Recombinant Human IL-4 (R&D Systems, Catalog # 204-IL) and Anti-Human IFN-gamma Antibody (R&D Systems, Catalog # AF-285-NA), followed by stimulation with 50 ng/mL PMA, 200 ng/mL calcium ionomycin, and 3 µM monensin for several hours. Live, single, CD4+ cells are shown in the dot plots, determined using a fixable viability dye, doublet exclusion, and staining with an Alexa Fluor® 700-conjugated Mouse Anti-Human CD4 Monoclonal Antibody (R&D Systems, Catalog # FAB3791N). The cells were additionally stained using a PE-conjugated Rat Anti-Human CCR3 Monoclonal Antibody (R&D Systems, Catalog # FAB155P), an Alexa Fluor® 647-conjugated Rabbit Anti-Human Phospho-STAT6 (Y641) Monoclonal Antibody (R&D Systems, Catalog # IC37173R), an Alexa Fluor® 594-conjugated Mouse Anti-Human GATA-3 Monoclonal Antibody (R&D Systems, Catalog # IC63301T), and an Alexa Fluor® 488-conjugated Rabbit Anti-Human IL-10 Monoclonal Antibody (R&D Systems, Catalog # IC9210G). Dot plots show the relative GATA-3+, Phospho-STAT6+, IL-10+, and CCR3+ cells in CD4+ resting (blue dots, lower left quadrant) and Th2-differentiated (orange dots, right quadrants) cell populations. Quadrant markers were set based on staining with the appropriate isotype controls (R&D Systems, Catalog # IC003N, # IC006P, IC1051R, # IC0041T, and # IC1051G). Note: The five fluorochrome-conjugated antibodies and buffers used for cell surface and intracellular staining of human Th2 cells using this protocol are also provided together in the FlowX™ Human Th2 Cell Multi-Color Flow Cytometry Kit (R&D Systems, Catalog # FMC011B).