Mouse CD45 Antibody Summary
Gln24-Lys425
Accession # NP_035340
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
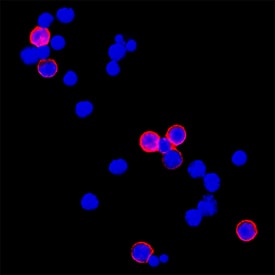
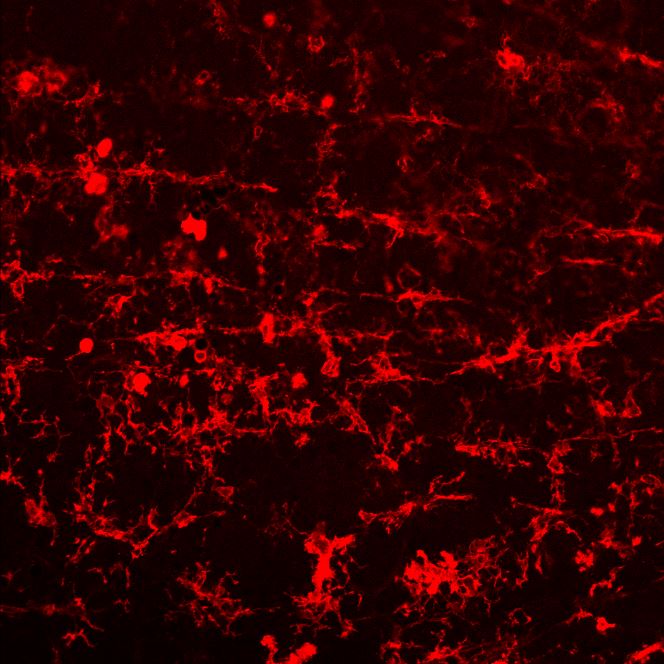
CD45 in RAW 264.7 Mouse Cell Line. CD45 was detected in immersion fixed RAW 264.7 mouse monocyte/macrophage cell line (positive staining) and Neuro‑2A mouse neuroblastoma cell line (negative staining) using Goat Anti-Mouse CD45 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF114) at 5 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; NL001) and counterstained with DAPI (blue). Specific staining was localized to cell surface. Staining was performed using our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
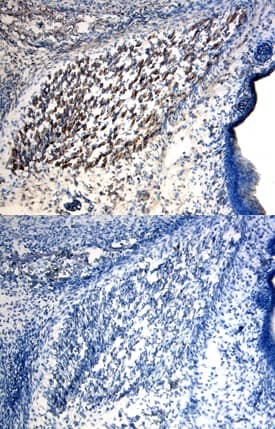
CD45 in Mouse Liver. CD45 was detected in immersion fixed paraffin-embedded sections of mouse liver using Goat Anti-Mouse CD45 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF114) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to Kupffer cells. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
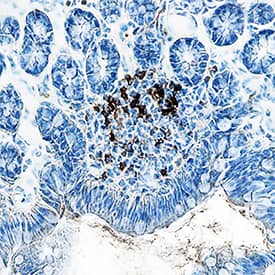
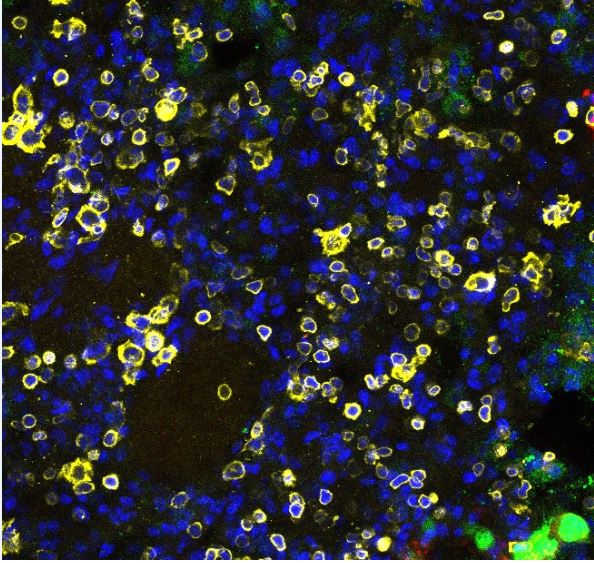
Detection of Mouse CD45 by Immunocytochemistry/Immunofluorescence The recruitment of CD45+F4/80+CD68+ macrophages was similar in the ASC-seeded DAT scaffolds that were cultured dynamically and statically for 14 days prior to implantation in the nu/nu mouse model. (A) Representative images showing CD45 (cyan), F4/80 (red), and CD68 (green) expression with DAPI counterstaining (blue) at 1-week post-implantation. Scale bars represent 100 μm. Boxed regions in the composite images are shown at higher magnification below. White arrowheads highlight CD45+F4/80+CD68+DAPI+ cells. (B) CD45+F4/80+CD68+DAPI+ cell density in the static and dynamic groups. (C) The percentage of CD45+F4/80+CD68+DAPI+ cells relative to the total CD45+DAPI+ cell population at 1, 4, and 8 weeks. *p < 0.05, **p < 0.01, ***p < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33816453), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CD45
Mouse CD45 (also known as Ly5 and leukocyte common antigen) is a 180‑220 kDa variably glycosylated member of the class 1 subtype of the protein tyrosine phosphatase family. It is synthesized as a 1291 amino acid (aa) precursor that contains a 23 aa signal sequence, a 541 aa extracellular domain (ECD), a 22 aa transmembrane segment, and a 705 aa cytoplasmic region. The ECD is coded for by exons 4‑16 of the CD45 gene. Alternate splicing of exon 4 (or A) (aa 30‑74), exon 5 (or B) (aa 75‑123) and exon 6 (or C) (aa 124‑169) define different lymphocyte populations and functional stages. Naïve T cells express exon 5 (CD45 RB), while activated T cells express neither exon 4, 5 or 6 (CD 45 RO). B cells express CD45 RABC, while resting NK cells express CD45 RA. Mouse CD45 ECD shares 60% and 44% aa sequence identity with rat and human full‑length CD45 ECD, respectively.
Product Datasheets
Citations for Mouse CD45 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
73
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Heterogeneity in macrophages along the cochlear spiral in mice: insights from SEM and functional analyses
Authors: Celia Zhang, Mengxiao Ye, Peter Bush, Bo Hua Hu
Frontiers in Cellular Neuroscience
-
RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis.
Authors: Yu M, Ting DT, Stott SL et al.
Nature
-
IL-17-induced HIF1? drives resistance to anti-PD-L1 via fibroblast-mediated immune exclusion
Authors: Chen X, Zhao J, Herjan T et al.
Journal of Experimental Medicine
-
Bacterial meningitis in the early postnatal mouse studied at single-cell resolution
Authors: Wang J, Rattner A, Nathans J
eLife
-
A Milieu Molecule for TGF-beta Required for Microglia Function in the Nervous System
Authors: Yan Qin, Brian S. Garrison, Wenjiang Ma, Rui Wang, Aiping Jiang, Jing Li et al.
Cell
-
Inhibition of fibronectin polymerization alleviates kidney injury due to ischemia-reperfusion
Authors: Stephanie L. K. Bowers, Stephanie Davis-Rodriguez, Zachary M. Thomas, Valeria Rudomanova, W. Clark Bacon, Alex Beiersdorfer et al.
American Journal of Physiology-Renal Physiology
-
Therapeutic activation of endothelial sphingosine‐1‐phosphate receptor 1 by chaperone‐bound S1P suppresses proliferative retinal neovascularization
Authors: Colin Niaudet, Bongnam Jung, Andrew Kuo, Steven Swendeman, Edward Bull, Takahiro Seno et al.
EMBO Molecular Medicine
-
Combining three independent pathological stressors induces a heart failure with preserved ejection fraction phenotype
Authors: Li Y, Kubo H, Yu D et al.
American journal of physiology. Heart and circulatory physiology
-
Inflammation and neutrophil extracellular traps in cerebral cavernous malformation
Authors: Anthony C. Y. Yau, Maria Ascencion Globisch, Favour Chinyere Onyeogaziri, Lei L. Conze, Ross Smith, Suvi Jauhiainen et al.
Cellular and Molecular Life Sciences
-
Renoprotective and Immunomodulatory Effects of GDF15 following AKI Invoked by Ischemia-Reperfusion Injury
Authors: Jing Liu, Sanjeev Kumar, Andreas Heinzel, Michael Gao, Jinjin Guo, Gregory F. Alvarado et al.
Journal of the American Society of Nephrology
-
Extracellular vesicle-dependent effect of RNA-binding protein IGF2BP1 on melanoma metastasis
Authors: Ghoshal, A;Rodrigues, LC;Gowda, CP;Elcheva, IA;Liu, Z;Abraham, T;Spiegelman, VS;
Oncogene
-
Polycomb Repressive Complex 2 promotes atherosclerotic plaque vulnerability
Authors: Joshi, D;Chakraborty, R;Bhogale, T;Furtado, J;Deng, H;Traylor, JG;Orr, AW;Martin, KA;Schwartz, MA;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Pervasive nuclear envelope ruptures precede ECM signaling and disease onset without activating cGAS-STING in Lamin-cardiomyopathy mice
Authors: En, A;Bogireddi, H;Thomas, B;Stutzman, A;Ikegami, S;LaForest, B;Almakki, O;Pytel, P;Moskowitz, I;Ikegami, K;
Cell Reports
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Oseltamivir (Tamiflu), a Commonly Prescribed Antiviral Drug, Mitigates Hearing Loss in Mice
Authors: Sailor-Longsworth, E;Lutze, RD;Ingersoll, MA;Kelmann, RG;Ly, K;Currier, D;Chen, T;Zuo, J;Teitz, T;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
mTORC1 hyperactivation and resultant suppression of macroautophagy contribute to the induction of cardiomyocyte necroptosis by catecholamine surges
Authors: Cai, M;Wu, P;Ni, W;Huang, D;Wang, X;
Physiological reports
Species: Rat
Sample Types: Cell Lysates
Applications: Western Blot -
Runx1+ vascular smooth muscle cells are essential for hematopoietic stem and progenitor cell development in vivo
Authors: Gonzalez Galofre, ZN;Kilpatrick, AM;Marques, M;Sá da Bandeira, D;Ventura, T;Gomez Salazar, M;Bouilleau, L;Marc, Y;Barbosa, AB;Rossi, F;Beltran, M;van de Werken, HJG;van IJcken, WFJ;Henderson, NC;Forbes, SJ;Crisan, M;
Nature communications
Species: Mouse
Sample Types: Embryo
Applications: IHC -
Innate and adaptive immune cell interaction drives inflammasome activation and hepatocyte apoptosis in murine liver injury from immune checkpoint inhibitors
Authors: Shojaie, L;Bogdanov, JM;Alavifard, H;Mohamed, MG;Baktash, A;Ali, M;Mahov, S;Murray, S;Kanel, GC;Liu, ZX;Ito, F;In, GK;Merchant, A;Stohl, W;Dara, L;
Cell death & disease
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Tunable PhenoCycler imaging of the murine pre-clinical tumour microenvironments
Authors: Abraham, MJ;Goncalves, C;McCallum, P;Gupta, V;Preston, SEJ;Huang, F;Chou, H;Gagnon, N;Johnson, NA;Miller, WH;Mann, KK;Del Rincon, SV;
Cell & bioscience
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Differential regulation of hair cell actin cytoskeleton mediated by SRF and MRTFB
Authors: Zhou, LY;Jin, CX;Wang, WX;Song, L;Shin, JB;Du, TT;Wu, H;
eLife
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunocytochemistry -
Cellular iron governs the host response to malaria
Authors: Wideman, SK;Frost, JN;Richter, FC;Naylor, C;Lopes, JM;Viveiros, N;Teh, MR;Preston, AE;White, N;Yusuf, S;Draper, SJ;Armitage, AE;Duarte, TL;Drakesmith, H;
PLoS pathogens
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Topical formulations of Aprepitant are safe and effective in relieving pain and inflammation, and drive neural regeneration
Authors: Bonelli, F;Demirsoy, I;Lasagni Vitar, RM;Fonteyne, P;Ferrari, G;
The ocular surface
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The cGAS-STING pathway is dispensable in a mouse model of LMNA -cardiomyopathy despite nuclear envelope rupture
Authors: En, A;Bogireddi, H;Thomas, B;Stutzman, A;Ikegami, S;LaForest, B;Almakki, O;Pytel, P;Moskowitz, IP;Ikegami, K;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and inflammation
Authors: Zhang, T;Xu, D;Liu, J;Wang, M;Duan, LJ;Liu, M;Meng, H;Zhuang, Y;Wang, H;Wang, Y;Lv, M;Zhang, Z;Hu, J;Shi, L;Guo, R;Xie, X;Liu, H;Erickson, E;Wang, Y;Yu, W;Dang, F;Guan, D;Jiang, C;Dai, X;Inuzuka, H;Yan, P;Wang, J;Babuta, M;Lian, G;Tu, Z;Miao, J;Szabo, G;Fong, GH;Karnoub, AE;Lee, YR;Pan, L;Kaelin, WG;Yuan, J;Wei, W;
Nature cell biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
SEC-seq: association of molecular signatures with antibody secretion in thousands of single human plasma cells
Authors: Cheng, RY;de Rutte, J;Ito, CEK;Ott, AR;Bosler, L;Kuo, WY;Liang, J;Hall, BE;Rawlings, DJ;Di Carlo, D;James, RG;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Capture -
Antibody-Mediated Delivery of VEGF-C Promotes Long-Lasting Lymphatic Expansion That Reduces Recurrent Inflammation
Authors: N Cousin, S Bartel, J Scholl, C Tacconi, A Egger, G Thorhallsd, D Neri, LC Dieterich, M Detmar
Cells, 2022-12-31;12(1):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Chronic suppurative otitis media causes macrophage-associated sensorineural hearing loss
Authors: A Xia, A Thai, Z Cao, X Chen, J Chen, B Bacacao, LA Bekale, V Schiel, PL Bollyky, PLS Maria
Journal of Neuroinflammation, 2022-09-12;19(1):224.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The protective effects of systemic dexamethasone on sensory epithelial damage and hearing loss in targeted Cx26-null mice
Authors: K Xu, S Chen, L Xie, Y Qiu, XZ Liu, X Bai, Y Jin, XH Wang, Y Sun
Cell Death & Disease, 2022-06-10;13(6):545.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
IL-17-induced HIF1? drives resistance to anti-PD-L1 via fibroblast-mediated immune exclusion
Authors: Chen X, Zhao J, Herjan T et al.
Journal of Experimental Medicine
-
Diabetes impairs cardioprotective function of endothelial progenitor cell-derived extracellular vesicles via H3K9Ac inhibition
Authors: G Huang, Z Cheng, A Hildebrand, C Wang, M Cimini, R Roy, AM Lucchese, C Benedict, V Mallaredy, A Magadum, D Joladarash, C Thej, C Gonzalez, M Trungcao, VNS Garikipati, JW Elrod, WJ Koch, R Kishore
Theranostics, 2022-05-21;12(9):4415-4430.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Tgf?1-Cthrc1 Signaling Plays an Important Role in the Short-Term Reparative Response to Heart Valve Endothelial Injury
Authors: Nordquist EM, Dutta P, Kodigepalli KM et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Dose and Dose Rate-Dependent Effects of Low-Dose Irradiation on Inflammatory Parameters in ApoE-Deficient and Wild Type Mice
Authors: A Glasow, I Patties, ND Priest, REJ Mitchel, G Hildebrand, K Manda
Cells, 2021-11-20;10(11):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
A transcriptome atlas of the mouse iris at single-cell resolution defines cell types and the genomic response to pupil dilation
Authors: J Wang, A Rattner, J Nathans
Elife, 2021-11-16;10(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth
Authors: Julia Gschwend, Samantha P.M. Sherman, Frederike Ridder, Xiaogang Feng, Hong-Erh Liang, Richard M. Locksley et al.
Journal of Experimental Medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Nociceptive sensory neurons promote CD8 T cell responses to HSV-1 infection
Authors: J Filtjens, A Roger, L Quatrini, E Wieduwild, J Gouilly, G Hoeffel, R Rossignol, C Daher, G Debroas, S Henri, CM Jones, B Malissen, LK Mackay, A Moqrich, FR Carbone, S Ugolini
Nature Communications, 2021-05-18;12(1):2936.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Intertumoral Genetic Heterogeneity Generates Distinct Tumor Microenvironments in a Novel Murine Synchronous Melanoma Model
Authors: SS Qin, BJ Han, A Williams, KM Jackson, R Jewell, AC Chacon, EM Lord, DC Linehan, M Kim, A Reuben, SA Gerber, PA Prieto
Cancers, 2021-05-11;13(10):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Modulating Ocular Surface Pain Through Neurokinin-1 Receptor Blockade
Authors: RM Lasagni Vi, M Barbariga, P Fonteyne, F Bignami, P Rama, G Ferrari
Investigative Ophthalmology & Visual Science, 2021-03-01;62(3):26.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Airway-Associated Macrophages in Homeostasis and Repair
Authors: AE Engler, AB Ysasi, RMF Pihl, C Villacorta, HM Heston, HMK Richardson, BR Thapa, NR Moniz, AC Belkina, SA Mazzilli, JR Rock
Cell Reports, 2020-12-29;33(13):108553.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Sectoral activation of glia in an inducible mouse model of autosomal dominant retinitis pigmentosa
Authors: MT Massengill, NF Ash, BM Young, CJ Ildefonso, AS Lewin
Sci Rep, 2020-10-12;10(1):16967.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The immune response after noise damage in the cochlea is characterized by a heterogeneous mix of adaptive and innate immune cells
Authors: V Rai, MB Wood, H Feng, NM Schabla, S Tu, J Zuo
Scientific Reports, 2020-09-16;10(1):15167.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Deficiency of peroxiredoxin 2 exacerbates angiotensin II-induced abdominal aortic aneurysm
Authors: SJ Jeong, MJ Cho, NY Ko, S Kim, IH Jung, JK Min, SH Lee, JG Park, GT Oh
Exp. Mol. Med., 2020-09-14;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Keratinocyte-Expressed Podoplanin is Dispensable for Multi-Step Skin Carcinogenesis
Authors: M Sesarti?, K Ikenberg, SY Yoon, M Detmar
Cells, 2020-06-24;9(6):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
FAM19A5 Expression During Embryogenesis and in the Adult Traumatic Brain of FAM19A5-LacZ Knock-in Mice
Authors: A Shahapal, EB Cho, HJ Yong, I Jeong, H Kwak, JK Lee, W Kim, B Kim, HC Park, WS Lee, H Kim, JI Hwang, JY Seong
Front Neurosci, 2019-08-30;13(0):917.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Antibody-mediated delivery of VEGF-C potently reduces chronic skin inflammation
Authors: S Schwager, S Renner, T Hemmerle, S Karaman, ST Proulx, R Fetz, AM Golding-Oc, P Probst, C Halin, D Neri, M Detmar
JCI Insight, 2018-12-06;3(23):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
STAT5A/B BLOCKADE SENSITIZES PROSTATE CANCER TO RADIATION THROUGH INHIBITION OF RAD51 AND DNA REPAIR
Authors: C Maranto, V Udhane, DT Hoang, L Gu, V Alexeev, KM Malas, K Cardenas, JR Brody, U Rodeck, C Bergom, KA Iczkowski, K Jacobsohn, WA See, SM Schmitt, MT Nevalainen
Clin. Cancer Res., 2018-02-26;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Type I interferons instigate fetal demise after Zika virus infection
Authors: LJ Yockey, KA Jurado, N Arora, A Millet, T Rakib, KM Milano, AK Hastings, E Fikrig, Y Kong, TL Horvath, S Weatherbee, HJ Kliman, CB Coyne, A Iwasaki
Sci Immunol, 2018-01-05;3(19):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion
Authors: J Liu, S Kumar, E Dolzhenko, GF Alvarado, J Guo, C Lu, Y Chen, M Li, MC Dessing, RK Parvez, PE Cippà, AM Krautzberg, G Saribekyan, AD Smith, AP McMahon
JCI Insight, 2017-09-21;2(18):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Prevention of C5aR1 signaling delays microglial inflammatory polarization, favors clearance pathways and suppresses cognitive loss
Authors: MX Hernandez, S Jiang, TA Cole, SH Chu, MI Fonseca, MJ Fang, LA Hohsfield, MD Torres, KN Green, RA Wetsel, A Mortazavi, AJ Tenner
Mol Neurodegener, 2017-09-18;12(1):66.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Metabolic origins of spatial organization in the tumor microenvironment
Authors: C Carmona-Fo, M Deforet, L Akkari, CB Thompson, JA Joyce, JB Xavier
Proc. Natl. Acad. Sci. U.S.A, 2017-02-28;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Immunization with Bacillus Calmette-Gu�rin (BCG) alleviates neuroinflammation and cognitive deficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain
Authors: Z Zuo, F Qi, J Yang, X Wang, Y Wu, Y Wen, Q Yuan, J Zou, K Guo, ZB Yao
Neurobiol. Dis, 2017-02-09;101(0):27-39.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
CREBH-FGF21 axis improves hepatic steatosis by suppressing adipose tissue lipolysis
Authors: Jong-Gil Park
Sci Rep, 2016-06-15;6(0):27938.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Low-dose irradiation affects expression of inflammatory markers in the heart of ApoE -/- mice.
Authors: Mathias D, Mitchel R, Barclay M, Wyatt H, Bugden M, Priest N, Whitman S, Scholz M, Hildebrandt G, Kamprad M, Glasow A
PLoS ONE, 2015-03-23;10(3):e0119661.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Runx1 is required for progression of CD41+ embryonic precursors into HSCs but not prior to this.
Authors: Liakhovitskaia A, Rybtsov S, Smith T, Batsivari A, Rybtsova N, Rode C, de Bruijn M, Buchholz F, Gordon-Keylock S, Zhao S, Medvinsky A
Development, 2014-09-01;141(17):3319-23.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Downstream Toll-like receptor signaling mediates adaptor-specific cytokine expression following focal cerebral ischemia.
J Neuroinflammation, 2012-07-16;9(0):174.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Serine protease inhibition reduces post-ischemic granulocyte recruitment in mouse intestine.
Authors: Gobbetti T, Cenac N, Motta JP, Rolland C, Martin L, Andrade-Gordon P, Steinhoff M, Barocelli E, Vergnolle N
Am. J. Pathol., 2011-11-07;180(1):141-52.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Protein-tyrosine phosphatase DEP-1 controls receptor tyrosine kinase FLT3 signaling.
Authors: Arora D, Stopp S, Bohmer SA, Schons J, Godfrey R, Masson K, Razumovskaya E, Ronnstrand L, Tanzer S, Bauer R, Bohmer FD, Muller JP
J. Biol. Chem., 2011-01-24;286(13):10918-29.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha.
Authors: Kraman M, Bambrough P, Arnold J, Roberts E, Magiera L, Jones J, Gopinathan A, Tuveson D, Fearon D
Science, 2010-11-05;330(6005):827-30.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Schistosomiasis-induced experimental pulmonary hypertension: role of interleukin-13 signaling.
Authors: Graham BB, Mentink-Kane MM, El-Haddad H
Am. J. Pathol., 2010-07-29;177(3):1549-61.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells.
Authors: Chou S, Lodish HF
Proc. Natl. Acad. Sci. U.S.A., 2010-04-12;107(17):7799-804.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages.
Authors: Tigges U, Hyer EG, Scharf J, Stallcup WB
Development, 2008-01-02;135(3):523-32.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
IL-12 alpha deficiency attenuates pressure overload-induced cardiac inflammation, hypertrophy, dysfunction, and heart failure progression
Authors: Umesh Bhattarai, Xiaochen He, Rui Xu, Xiaoguang Liu, Lihong Pan, Yuxiang Sun et al.
Frontiers in Immunology
-
Promotion of Lymphangiogenesis by Targeted Delivery of VEGF-C Improves Diabetic Wound Healing
Authors: LM Brunner, Y He, N Cousin, J Scholl, LK Albin, B Schmucki, S Supersaxo, G Restivo, J Hafner, D Neri, S Werner, M Detmar
Cells, 2023-02-01;12(3):.
-
Automated segmentation and analysis of retinal microglia within ImageJ
Authors: Neil F. Ash, Michael T. Massengill, Lindsey Harmer, Ahmed Jafri, Alfred S. Lewin
Experimental Eye Research
-
Tgf?1-Cthrc1 Signaling Plays an Important Role in the Short-Term Reparative Response to Heart Valve Endothelial Injury
Authors: Nordquist EM, Dutta P, Kodigepalli KM et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response
Authors: Juan Carlos Pena-Philippides, Ernesto Caballero-Garrido, Tamar Lordkipanidze, Tamara Roitbak
Journal of Neuroinflammation
-
Active immunization against complement factor C5a: a new therapeutic approach for Alzheimer’s disease
Authors: Christine Landlinger, Lisa Oberleitner, Petra Gruber, Birgit Noiges, Kristyna Yatsyk, Radmila Santic et al.
Journal of Neuroinflammation
-
Molecular profile of cochlear immunity in the resident cells of the organ of Corti
Authors: Qunfeng Cai, R Robert Vethanayagam, Shuzhi Yang, Jonathan Bard, Jennifer Jamison, Daniel Cartwright et al.
Journal of Neuroinflammation
-
Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease
Authors: Caitlin M. Braitsch, Onur Kanisicak, Jop H. van Berlo, Jeffery D. Molkentin, Katherine E. Yutzey
Journal of Molecular and Cellular Cardiology
-
Growth and maturation of heart valves leads to changes in endothelial cell distribution, impaired function, decreased metabolism and reduced cell proliferation
Authors: Lindsey J. Anstine, Chris Bobba, Samir Ghadiali, Joy Lincoln
Journal of Molecular and Cellular Cardiology
-
Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function
Authors: Epameinondas Gousopoulos, Steven T. Proulx, Samia B. Bachmann, Jeannette Scholl, Dimitris Dionyssiou, Efterpi Demiri et al.
JCI Insight
-
Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth
Authors: Julia Gschwend, Samantha P.M. Sherman, Frederike Ridder, Xiaogang Feng, Hong-Erh Liang, Richard M. Locksley et al.
Journal of Experimental Medicine
-
Contribution of Extra-Cardiac Cells in Murine Heart Valves is Age-Dependent.
Authors: Anstine LJ, Horne TE, Horwitz EM, Lincoln J.
J Am Heart Assoc
-
Local Macrophage-Related Immune Response Is Involved in Cochlear Epithelial Damage in Distinct Gjb2-Related Hereditary Deafness Models
Authors: Xu K, Chen S, Xie L, et al.
Frontiers in cell and developmental biology
-
Endothelial Cell Lineage Analysis Does Not Provide Evidence for EMT in Adult Valve Homeostasis and Disease
Authors: Andrew J. Kim, Christina M. Alfieri, Katherine E. Yutzey
The Anatomical Record
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsIsotype Controls
Reconstitution Buffers
Secondary Antibodies
Reviews for Mouse CD45 Antibody
Average Rating: 5 (Based on 2 Reviews)
Have you used Mouse CD45 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
DAPI (Blue)
CD45 (Yellow)