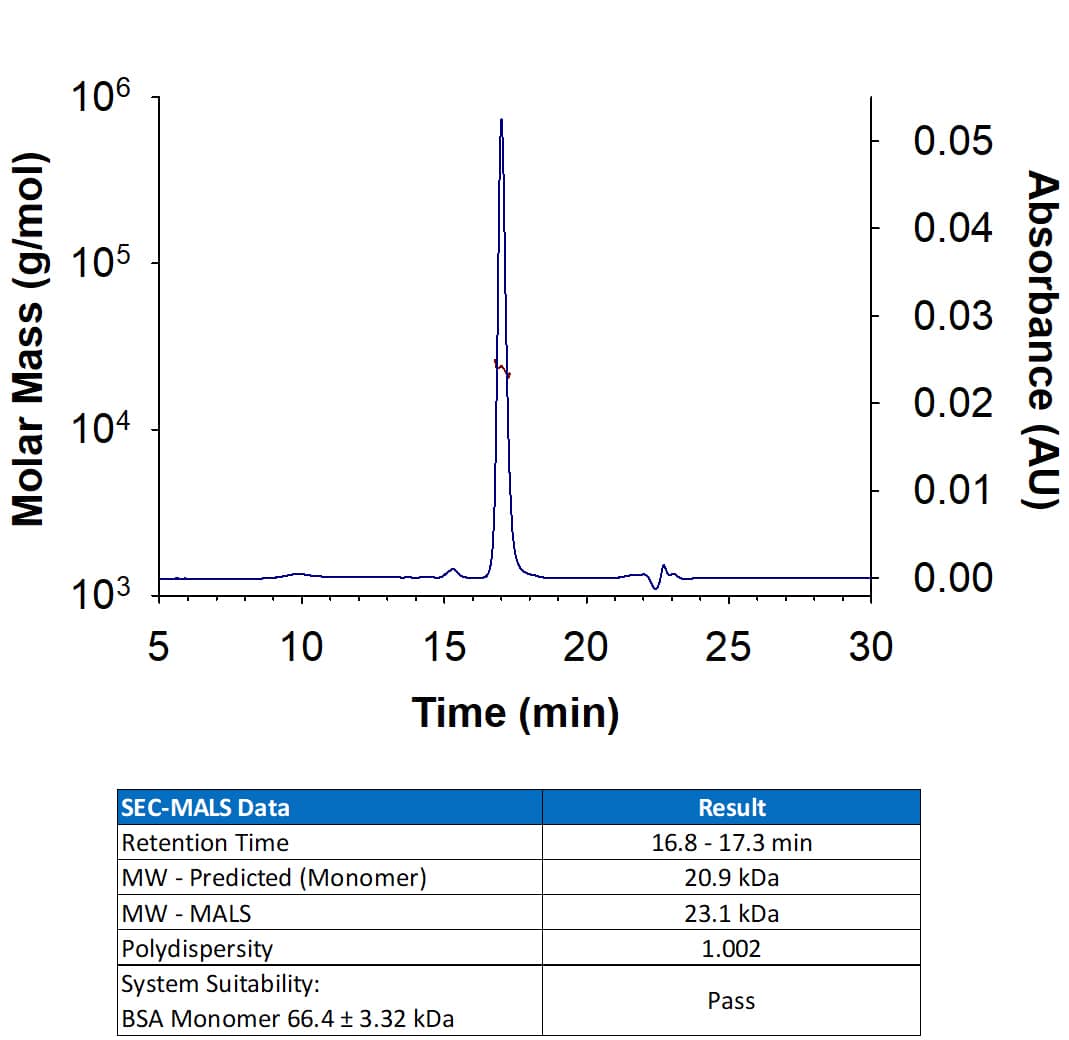
Human IL-6 Antibody Summary
Pro29-Met212
Accession # P05231
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
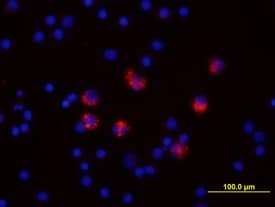
IL‑6 in Human PBMCs. IL-6 was detected in immersion fixed human peripheral blood mononuclear cells (PBMCs) treated with LPS and monensin using Goat Anti-Human IL-6 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-206-NA) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counter-stained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
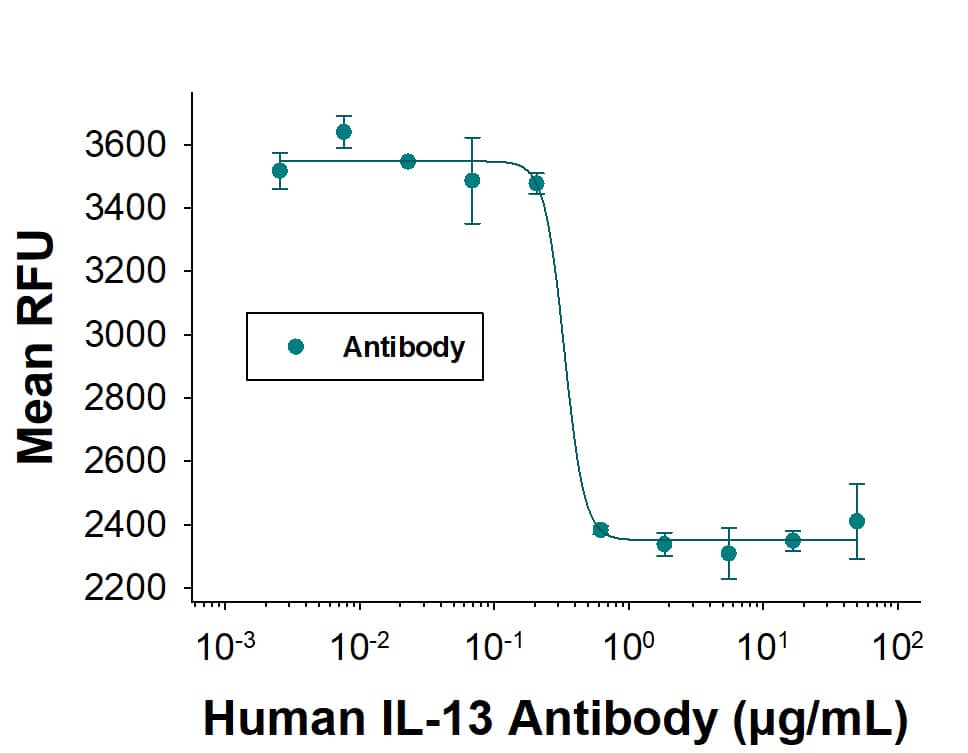
Cell Proliferation Induced by IL‑6 and Neutralization by Human IL‑6 Antibody. Recombinant Human IL-6 (Catalog # 206-IL) stimulates proliferation in the T1165.85.2.1 mouse plasmacytoma cell line in a dose-dependent manner (orange line). Proliferation elicited by Recombinant Human IL-6 (2.5 ng/mL) is neutralized (green line) by increasing concentrations of Goat Anti-Human IL-6 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-206-NA). The ND50 is typically ≤ 125 ng/mL.
 View Larger
View Larger
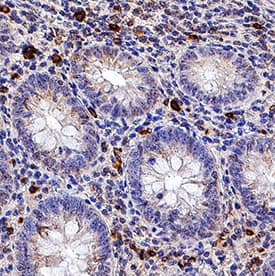
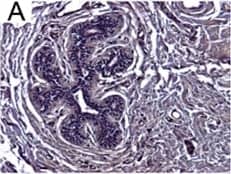
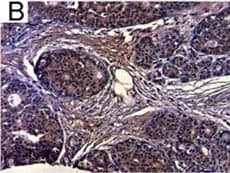
Detection of Human IL-6 by Immunohistochemistry Breast DCIS cells overexpress proinflammatory markers. Representative images of immunohistochemistry targeting IL-6 protein in normal breast tissue (a), and breast DCIS (b) (N = 61). c and d Hematoxylin staining of serial sections from tissue shown in panel A and B. All images are 20X magnification. Scale bar equals 100 μm. e Evaluation of IL-6 gene expression in DCIS cells via qRT-PCR; the isogenic MCF10.DCIS cells and the non-isogenic SUM102 cell line were analyzed against the non-transformed MCF10A cell line (N = 3). f Secretion of IL-6 protein from DCIS cell lines and non-transformed MCF10A cells as determined by ELISA. *P < 0.05, Student’s t-test; mean ± SD Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26268945), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
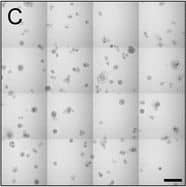
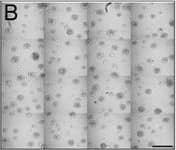
Detection of Human IL-6 by Block/Neutralize IL-6 neutralizing antibody (nAb) inhibits growth of MCF10.DCIS structures. Representative contiguous 16-tiled DIC images of MCF10.DCIS cells grown in MAME cultures for 8 days. MAME culture of MCF10.DCIS cells treated with an isotype-matched antibody against IgG (control) (8 days) (a), or 1 μg /ml IL-6 nAb (b) (8 days). MCF10.DCIS cells treated with IL-6 nAb followed by a 48 h treatment-free recovery period prior to imaging on day 10 (c). N = 3, Scale bars, 200 μm. d Diameter of MCF10.DCIS structures in the presence of control antibody, IL-6 nAb, or 48 h recovery from IL-6 nAb. N = 20-40 measurements /tiled DIC image (N = 3). ****P ≤ 0.0001, **P ≤ 0.01. e Evaluation of gene expression of invasive tumor cell markers in MAME cultures treated with IL-6 nAb (N = 3). Fold difference as compared to control cultures. Dashed line indicates 2-fold threshold. Student’s t-test; mean ± SD Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26268945), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human IL-6 by Block/Neutralize IL-6 nAb inhibits CAF-stimulated MCF10.DCIS structure growth and results in altered morphology. Contiguous 16-tile DIC image of CAFs: WS12Ti (A, B) or CAF40TKi (c, d) co-cultured with MCF10.DCIS cells for 8 days in the presence of isotype matched anti-IgG (a, c) or 1 μg /ml IL-6 nAb (b, d). e Perimeter measurements from DCIS structures in MAME culture in the presence of anti-IgG or IL-6 nAb (N = 100-140 measurements from 6 contiguous tiled DIC images (N = 3). Fluorescent tiled images of CAF40TKi fibroblasts (unlabeled) cultured with MCF10.DCIS-RFP cells in the presence of anti-IgG (f) or 1 μg /ml IL-6 nAb (g). h Quantification of total volume of DCIS-RFP structures in co-culture with CAF40TKi fibroblasts (ratio-paired t-test, N = 3). ***P ≤ 0.001, **P ≤ 0.01; Student’s t-test; mean ± SD Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26268945), licensed under a CC-BY license. Not internally tested by R&D Systems.
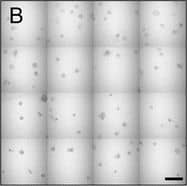 View Larger
View Larger
Detection of Human IL-6 by Block/Neutralize IL-6 neutralizing antibody (nAb) inhibits growth of MCF10.DCIS structures. Representative contiguous 16-tiled DIC images of MCF10.DCIS cells grown in MAME cultures for 8 days. MAME culture of MCF10.DCIS cells treated with an isotype-matched antibody against IgG (control) (8 days) (a), or 1 μg /ml IL-6 nAb (b) (8 days). MCF10.DCIS cells treated with IL-6 nAb followed by a 48 h treatment-free recovery period prior to imaging on day 10 (c). N = 3, Scale bars, 200 μm. d Diameter of MCF10.DCIS structures in the presence of control antibody, IL-6 nAb, or 48 h recovery from IL-6 nAb. N = 20-40 measurements /tiled DIC image (N = 3). ****P ≤ 0.0001, **P ≤ 0.01. e Evaluation of gene expression of invasive tumor cell markers in MAME cultures treated with IL-6 nAb (N = 3). Fold difference as compared to control cultures. Dashed line indicates 2-fold threshold. Student’s t-test; mean ± SD Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26268945), licensed under a CC-BY license. Not internally tested by R&D Systems.
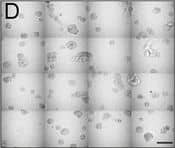 View Larger
View Larger
Detection of Human IL-6 by Block/Neutralize IL-6 nAb inhibits CAF-stimulated MCF10.DCIS structure growth and results in altered morphology. Contiguous 16-tile DIC image of CAFs: WS12Ti (A, B) or CAF40TKi (c, d) co-cultured with MCF10.DCIS cells for 8 days in the presence of isotype matched anti-IgG (a, c) or 1 μg /ml IL-6 nAb (b, d). e Perimeter measurements from DCIS structures in MAME culture in the presence of anti-IgG or IL-6 nAb (N = 100-140 measurements from 6 contiguous tiled DIC images (N = 3). Fluorescent tiled images of CAF40TKi fibroblasts (unlabeled) cultured with MCF10.DCIS-RFP cells in the presence of anti-IgG (f) or 1 μg /ml IL-6 nAb (g). h Quantification of total volume of DCIS-RFP structures in co-culture with CAF40TKi fibroblasts (ratio-paired t-test, N = 3). ***P ≤ 0.001, **P ≤ 0.01; Student’s t-test; mean ± SD Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26268945), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human IL-6 by Immunohistochemistry Breast DCIS cells overexpress proinflammatory markers. Representative images of immunohistochemistry targeting IL-6 protein in normal breast tissue (a), and breast DCIS (b) (N = 61). c and d Hematoxylin staining of serial sections from tissue shown in panel A and B. All images are 20X magnification. Scale bar equals 100 μm. e Evaluation of IL-6 gene expression in DCIS cells via qRT-PCR; the isogenic MCF10.DCIS cells and the non-isogenic SUM102 cell line were analyzed against the non-transformed MCF10A cell line (N = 3). f Secretion of IL-6 protein from DCIS cell lines and non-transformed MCF10A cells as determined by ELISA. *P < 0.05, Student’s t-test; mean ± SD Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26268945), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: IL-6
Interleukin-6 (IL-6) is a pleiotropic, alpha -helical, phosphorylated and variably glycosylated cytokine that plays important roles in the acute phase reaction, inflammation, hematopoiesis, bone metabolism, and cancer progression. Mature human IL-6 is 183 amino acids (aa) in length expressed as a 22-28 kDA molecular weight protein. IL-6 shares 39% aa sequence identity with mouse and rat IL-6. Alternative splicing generates several isoforms with internal deletions, some of which exhibit antagonistic properties. IL-6 induces signaling through a cell surface heterodimeric receptor complex composed of a ligand binding subunit (IL-6 R alpha) and a signal transducing subunit (gp130). IL-6 binds to IL-6 R alpha, triggering IL-6 R alpha association with gp130 and gp130 dimerization. gp130 is also a component of the receptors for CLC, CNTF, CT-1, IL-11, IL-27, LIF, and OSM. Soluble forms of IL-6 R alpha are generated by both alternative splicing and proteolytic cleavage. In a mechanism known as trans-signaling, complexes of soluble IL-6 and IL-6 R alpha elicit responses from gp130-expressing cells that lack cell surface IL-6 R alpha. Trans-signaling enables a wider range of cell types to respond to IL-6, as the expression of gp130 is ubiquitous, while that of IL-6 R alpha is predominantly restricted to hepatocytes, monocytes, and resting lymphocytes. Soluble splice forms of gp130 block trans-signaling from IL-6/IL-6 R alpha but not from other cytokines that use gp130 as a co-receptor. IL-6, along with TNF-alpha and IL-1, function to drive the acute inflammatory response and the transition from acute inflammation to either acquired immunity or chronic inflammatory disease. When dysregulated, it contributes to chronic inflammation in obesity, insulin resistance, inflammatory bowel disease, arthritis, sepsis, and atherosclerosis. IL-6 can also function as an anti-inflammatory molecule, as in skeletal muscle where it is secreted in response to exercise. In addition, it enhances hematopoietic stem cell proliferation and the differentiation of Th17 cells, memory B cells, and plasma cells.
Product Datasheets
Citations for Human IL-6 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
52
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging
Authors: MP Baar, RM Brandt, DA Putavet, JD Klein, KW Derks, BR Bourgeois, S Stryeck, Y Rijksen, H van Willig, DA Feijtel, I van der Pl, J Essers, WA van Cappel, WF van IJcken, AB Houtsmulle, J Pothof, RW de Bruin, T Madl, JH Hoeijmaker, J Campisi, PL de Keizer
Cell, 2017-03-23;169(1):132-147.e16.
-
Post-acute sequelae of SARS-CoV-2 cardiovascular symptoms are associated with trace-level cytokines that affect cardiomyocyte function
Authors: Sinclair, JE;Vedelago, C;Ryan, FJ;Carney, M;Redd, MA;Lynn, MA;Grubor-Bauk, B;Cao, Y;Henders, AK;Chew, KY;Gilroy, D;Greaves, K;Labzin, L;Ziser, L;Ronacher, K;Wallace, LM;Zhang, Y;Macauslane, K;Ellis, DJ;Rao, S;Burr, L;Bain, A;Karawita, A;Schulz, BL;Li, J;Lynn, DJ;Palpant, N;Wuethrich, A;Trau, M;Short, KR;
Nature microbiology
Species: Human
Sample Types: Plasma
Applications: Bioassay -
IL-33-dependent NF-?B activation inhibits apoptosis and drives chemoresistance in acute myeloid leukemia
Authors: Yan, M;Chen, X;Ye, Q;Li, H;Zhang, L;Wang, Y;
Cytokine
Species: Human
Sample Types: Protein
Applications: Western Blot -
Unravelling heterogeneous effects of cancer?associated fibroblasts on poor prognosis markers in breast cancer EM?G3 cell line: In vitro?targeted treatment (anti?IL-6, anti?VEGF-A, anti?MFGE8) based on transcriptomic profiling
Authors: Urban, L;Novák, ;?oma, M;Dvo?ánková, B;Lacina, L;áchová, J;Hradilová, M;Svato?ová, P;Kolá?, M;Strnad, H;B?ezinová, J;Smetana, K;Gál, P;Szabo, P;
Oncology reports
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Leptin-Mediated Induction of IL-6 Expression in Hofbauer Cells Contributes to Preeclampsia Pathogenesis
Authors: Ozmen, A;Nwabuobi, C;Tang, Z;Guo, X;Larsen, K;Guller, S;Blas, J;Moore, M;Kayisli, UA;Lockwood, CJ;Guzeloglu-Kayisli, O;
International journal of molecular sciences
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Suppression of TGF beta -Induced Interleukin-6 Secretion by Sinulariolide from Soft Corals through Attenuation of the p38-NF-kB Pathway in Carcinoma Cells
Authors: Yang, JL;Lin, WL;Tai, SB;Ciou, YS;Chung, CL;Chen, JJ;Liu, PF;Lin, MW;Chen, CL;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
Applications: Western Blot -
Extending the dynamic range of biomarker quantification through molecular equalization
Authors: Newman, S;Wilson, B;Mamerow, D;Wollant, B;Nyein, H;Rosenberg-Hasson, Y;Maecker, H;Eisenstein, M;Soh, H;
bioRxiv
Species: Human
Sample Types: Serum
Applications: Bioassay -
CCL18 signaling from tumor-associated macrophages activates fibroblasts to adopt a chemoresistance-inducing phenotype
Authors: W Zeng, L Xiong, W Wu, S Li, J Liu, L Yang, L Lao, P Huang, M Zhang, H Chen, N Miao, Z Lin, Z Liu, X Yang, J Wang, P Wang, E Song, Y Yao, Y Nie, J Chen, D Huang
Oncogene, 2022-11-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, Neutralization -
Effects of interleukin 1beta on long noncoding RNA and mRNA expression profiles of human synovial fluid derived mesenchymal stem cells
Authors: YP Sun, YY Lu, J Chen, JH Bao, H Zhang, JD Sun, WT Liao
Scientific Reports, 2022-05-19;12(1):8432.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Pro-Inflammatory Cytokines Trigger the Overexpression of Tumour-Related Splice Variant RAC1B in Polarized Colorectal Cells
Authors: JFS Pereira, C Bessa, P Matos, P Jordan
Cancers, 2022-03-09;14(6):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Proximity Extension Assay in Combination with Next-Generation Sequencing for High-throughput Proteome-wide Analysis
Authors: Lotta Wik, Niklas Nordberg, John Broberg, Johan Björkesten, Erika Assarsson, Sara Henriksson et al.
Molecular & Cellular Proteomics
-
Inhibition of relaxin autocrine signaling confers therapeutic vulnerability in ovarian cancer
Authors: Helen E. Burston, Oliver A. Kent, Laudine Communal, Molly L. Udaskin, Ren X. Sun, Kevin R. Brown et al.
Journal of Clinical Investigation
-
Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation
Authors: Q Ji, L Zhou, H Sui, L Yang, X Wu, Q Song, R Jia, R Li, J Sun, Z Wang, N Liu, Y Feng, X Sun, G Cai, Y Feng, J Cai, Y Cao, G Cai, Y Wang, Q Li
Nat Commun, 2020-03-05;11(1):1211.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
A homogeneous bioluminescent immunoassay to probe cellular signaling pathway regulation
Authors: Byounghoon (Brian) Hwang, Laurie Engel, Said A. Goueli, Hicham Zegzouti
Communications Biology
-
Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging
Authors: DP Muñoz, SM Yannone, A Daemen, Y Sun, F Vakar-Lope, M Kawahara, AM Freund, F Rodier, JD Wu, PY Desprez, DH Raulet, PS Nelson, LJ van 't Vee, J Campisi, JP Coppé
JCI Insight, 2019-06-11;5(0):.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Severe Leptospirosis Features in the Spleen Indicate Cellular Immunosuppression Similar to That Found in Septic Shock
Authors: Amaro Nunes Duarte-Neto, Julio Croda, Carla Pagliari, Francisco Garcia Soriano, Antonio Carlos Nicodemo, Maria Irma Seixas Duarte
Frontiers in Immunology
-
Osteoblasts are "educated" by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment
Authors: AD Kolb, AB Shupp, D Mukhopadhy, FC Marini, KM Bussard
Breast Cancer Res., 2019-02-27;21(1):31.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-P -
Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice
Authors: Y Yin, H Hao, Y Cheng, L Zang, J Liu, J Gao, J Xue, Z Xie, Q Zhang, W Han, Y Mu
Cell Death Dis, 2018-07-09;9(7):760.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Improved efficiency of in situ protein analysis by proximity ligation using UnFold probes
Authors: A Klaesson, K Grannas, T Ebai, J Heldin, B Koos, M Leino, D Raykova, J Oelrich, L Arngården, O Söderberg, U Landegren
Sci Rep, 2018-03-29;8(1):5400.
Species: Human
Sample Types: Recombinant Protein
Applications: Proximity Ligation Assay (PLA) -
Increased interleukin-17A levels promote rituximab resistance by suppressing p53 expression and predict an unfavorable prognosis in patients with diffuse large B cell lymphoma
Authors: Weijie Zhong, Xin Xu, Zhigang Zhu, Li Yang, Hong Du, Zhongjun Xia et al.
International Journal of Oncology
-
Streamlined circular proximity ligation assay provides high stringency and compatibility with low-affinity antibodies
Authors: R Jalili, J Horecka, JR Swartz, RW Davis, HHJ Persson
Proc. Natl. Acad. Sci. U.S.A., 2018-01-16;0(0):.
Applications: Affinity Assay -
Activation of adrenergic receptor beta 2 promotes tumor progression and epithelial mesenchymal transition in tongue squamous cell carcinoma
Authors: Haichao Liu, Cheng Wang, Nan Xie, Zehang Zhuang, Xiqiang Liu, Jinsong Hou et al.
International Journal of Molecular Medicine
-
SorLA in interleukin-6 signaling and turnover
Authors: JV Larsen, CM Petersen
Mol. Cell. Biol, 2017-05-16;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Pathomimetic avatars reveal divergent roles of microenvironment in invasive transition of ductal carcinoma in situ
Authors: M Sameni, D Cavallo-Me, OE Franco, A Chalasani, K Ji, N Aggarwal, A Anbalagan, X Chen, RR Mattingly, SW Hayward, BF Sloane
Breast Cancer Res., 2017-05-15;19(1):56.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Adipose Stromal Cells from Visceral and Subcutaneous Fat Facilitate Migration of Ovarian Cancer Cells via IL-6/JAK2/STAT3 Pathway
Authors: B Kim, HS Kim, S Kim, G Haegeman, BK Tsang, DN Dhanasekar, YS Song
Cancer Res Treat, 2016-07-18;49(2):338-349.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation.
Authors: Procopio M, Laszlo C, Al Labban D, Kim D, Bordignon P, Jo S, Goruppi S, Menietti E, Ostano P, Ala U, Provero P, Hoetzenecker W, Neel V, Kilarski W, Swartz M, Brisken C, Lefort K, Dotto G
Nat Cell Biol, 2015-08-24;17(9):1193-204.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Il-6 signaling between ductal carcinoma in situ cells and carcinoma-associated fibroblasts mediates tumor cell growth and migration.
Authors: Osuala K, Sameni M, Shah S, Aggarwal N, Simonait M, Franco O, Hong Y, Hayward S, Behbod F, Mattingly R, Sloane B
BMC Cancer, 2015-08-13;15(0):584.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: IHC, Neutralization -
Stromal TGF-beta signaling induces AR activation in prostate cancer
Authors: Feng Yang, Yizhen Chen, Tao Shen, Dan Guo, Olga Dakhova, Michael M. Ittmann et al.
Oncotarget
-
Fucose-based PAMPs prime dendritic cells for follicular T helper cell polarization via DC-SIGN-dependent IL-27 production.
Authors: Gringhuis S, Kaptein T, Wevers B, van der Vlist M, Klaver E, van Die I, Vriend L, de Jong M, Geijtenbeek T
Nat Commun, 2014-10-03;5(0):5074.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Phosphoproteomic profiling reveals IL6-mediated paracrine signaling within the Ewing sarcoma family of tumors.
Authors: Anderson J, Titz B, Akiyama R, Komisopoulou E, Park A, Tap W, Graeber T, Denny C
Mol Cancer Res, 2014-08-04;12(12):1740-54.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Tumor-activated TCRgammadelta(+) T cells from gastric cancer patients induce the antitumor immune response of TCRalphabeta(+) T cells via their antigen-presenting cell-like effects.
Authors: Mao C, Mou X, Zhou Y, Yuan G, Xu C, Liu H, Zheng T, Tong J, Wang S, Chen D
J Immunol Res, 2014-03-31;2014(0):593562.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Digital microfluidic assay for protein detection.
Authors: Mok J, Mindrinos M, Davis R, Javanmard M
Proc Natl Acad Sci U S A, 2014-01-21;111(6):2110-5.
Species: N/A
Sample Types: Recombinant Protein
Applications: ELISA Capture, ELISA Detection -
Inhibition of IL-6 expression in LNCaP prostate cancer cells by a combination of atorvastatin and celecoxib.
Authors: Wang H, Cui X, Goodin S, Ding N, Van Doren J, Du Z, Huang M, Liu Y, Cheng X, Dipaola R, Conney A, Zheng X
Oncol Rep, 2013-11-29;31(2):835-41.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Microspectroscopic SERS detection of interleukin-6 with rationally designed gold/silver nanoshells
Authors: Yuling Wang, Mohammad Salehi, Max Schütz, Katharina Rudi, Sebastian Schlücker
The Analyst
-
Pre-analytical effects of blood sampling and handling in quantitative immunoassays for rheumatoid arthritis.
Authors: Zhao X, Qureshi F, Eastman PS, Manning WC, Alexander C, Robinson WH, Hesterberg LK
J. Immunol. Methods, 2012-02-17;378(1):72-80.
Species: Human
Sample Types: Serum
Applications: ELISA Development -
IL-6 Promotes Head and Neck Tumor Metastasis by Inducing Epithelial–Mesenchymal Transition via the JAK-STAT3-SNAIL Signaling Pathway
Authors: Arti Yadav, Bhavna Kumar, Jharna Datta, Theodoros N. Teknos, Pawan Kumar
Molecular Cancer Research
-
PLTP regulates STAT3 and NF kappa B in differentiated THP1 cells and human monocyte-derived macrophages
Authors: S. Vuletic, W. Dong, G. Wolfbauer, C. Tang, J.J. Albers
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research
-
Microarray and Proteomic Analysis of Breast Cancer Cell and Osteoblast Co-cultures: ROLE OF OSTEOBLAST MATRIX METALLOPROTEINASE (MMP)-13 IN BONE METASTASIS.
Authors: Morrison C, Mancini S, Cipollone J, Kappelhoff R, Roskelley C, Overall C
J. Biol. Chem., 2011-07-22;286(39):34271-85.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Western Blot -
Bone marrow stroma-secreted cytokines protect JAK2(V617F)-mutated cells from the effects of a JAK2 inhibitor
Authors: Taghi Manshouri, Zeev Estrov, Alfonso Quintás-Cardama, Jan Burger, Ying Zhang, Ana Livun et al.
Cancer Research
-
p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype.
Authors: Freund A, Patil CK, Campisi J
EMBO J., 2011-03-11;30(8):1536-48.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells.
Authors: Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, Groshen SG, Seeger RC, DeClerck YA
Cancer Res., 2009-01-01;69(1):329-37.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production.
Authors: Ahmed S, Marotte H, Kwan K, Ruth JH, Campbell PL, Rabquer BJ, Pakozdi A, Koch AE
Proc. Natl. Acad. Sci. U.S.A., 2008-09-16;105(38):14692-7.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network.
Authors: Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS
Cell, 2008-06-13;133(6):1019-31.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Exploring human testicular peritubular cells: identification of secretory products and regulation by tumor necrosis factor-alpha.
Authors: Schell C, Albrecht M, Mayer C, Schwarzer JU, Frungieri MB, Mayerhofer A
Endocrinology, 2008-01-10;149(4):1678-86.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Bronchial epithelial cell growth regulation in fibroblast cocultures: the role of hepatocyte growth factor.
Authors: Skibinski G, Elborn JS, Ennis M
Am. J. Physiol. Lung Cell Mol. Physiol., 2007-03-23;293(1):L69-76.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
T cell-dependent survival of CD20+ and CD20- plasma cells in human secondary lymphoid tissue.
Authors: Withers DR, Fiorini C, Fischer RT, Ettinger R, Lipsky PE, Grammer AC
Blood, 2007-02-13;109(11):4856-64.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Interleukin-6 and vascular endothelial growth factor release by renal cell carcinoma cells impedes lymphocyte-dendritic cell cross-talk.
Authors: Cabillic F, Bouet-Toussaint F, Toutirais O, Rioux-Leclercq N, Fergelot P, de La Pintiere CT, Genetet N, Patard JJ, Catros-Quemener V
Clin. Exp. Immunol., 2006-12-01;146(3):518-23.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Induction of interleukin-6 by hepatitis C virus core protein in hepatitis C-associated mixed cryoglobulinemia and B-cell non-Hodgkin's lymphoma.
Authors: Feldmann G, Nischalke HD, Nattermann J, Banas B, Berg T, Teschendorf C, Schmiegel W, Duhrsen U, Halangk J, Iwan A, Sauerbruch T, Caselmann WH, Spengler U
Clin. Cancer Res., 2006-08-01;12(15):4491-8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Normalizing the bone marrow microenvironment with p38 inhibitor reduces multiple myeloma cell proliferation and adhesion and suppresses osteoclast formation.
Authors: Nguyen AN, Stebbins EG, Henson M, O&apos;Young G, Choi SJ, Quon D, Damm D, Reddy M, Ma JY, Haghnazari E, Kapoun AM, Medicherla S, Protter A, Schreiner GF, Kurihara N, Anderson J, Roodman GD, Navas TA, Higgins LS
Exp. Cell Res., 2006-04-04;312(10):1909-23.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Influence of eicosapentaenoic acid, an omega-3 fatty acid, on ultraviolet-B generation of prostaglandin-E2 and proinflammatory cytokines interleukin-1 beta, tumor necrosis factor-alpha, interleukin-6 and interleukin-8 in human skin in vivo.
Authors: Shahbakhti H, Watson RE, Azurdia RM, Ferreira CZ, Garmyn M, Rhodes LE
Photochem. Photobiol., 2004-09-01;80(2):231-5.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Immunohistochemical localization of leukemia inhibitory factor, interleukins 1 and 6 at the primary implantation site in the rhesus monkey.
Authors: Sengupta J, Dhawan L, Ghosh D
Cytokine, 2003-12-21;24(6):277-85.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC-P -
Arenavirus-mediated liver pathology: acute lymphocytic choriomeningitis virus infection of rhesus macaques is characterized by high-level interleukin-6 expression and hepatocyte proliferation.
Authors: Lukashevich IS, Tikhonov I, Rodas JD, Zapata JC, Yang Y, Djavani M, Salvato MS
J. Virol., 2003-02-01;77(3):1727-37.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Neutralization
FAQs
-
What band size does AF-206-NA detect in Western blot?
AF-206-NA detects a 20-22 kDa band in lysate in Western blot.
Isotype Controls
Reconstitution Buffers
Secondary Antibodies
Reviews for Human IL-6 Antibody
Average Rating: 5 (Based on 2 Reviews)
Have you used Human IL-6 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
After labeling with Sulfo-Tag, used as a detection reagent in MSD assay (Meso Scale Diagnostics LLC). Paired with biotinylated IL-6 antibody (MAB206) as a capture reagent. A standard curve with recombinant human IL-6 from (Cat# 206-IL/CF) is shown (1.6-25,000 pg/ml).