Human/Mouse E-Cadherin Antibody Summary
Asp157-Val709
Accession # P09803
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
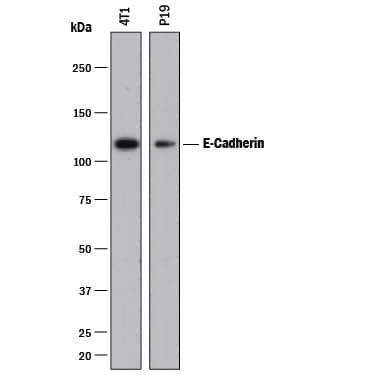
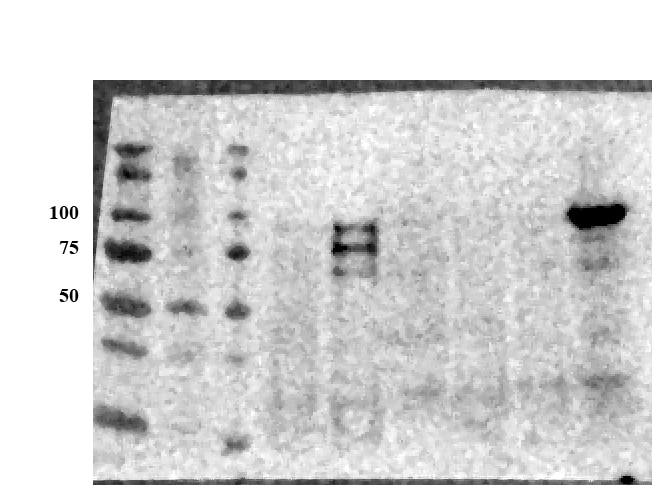
Detection of Human and Mouse E‑Cadherin by Western Blot. Western blot shows lysates of A431 human epithelial carcinoma cell line, A549 human lung carcinoma cell line, HepG2 human hepatocellular carcinoma cell line, P19 mouse embryonal carcinoma cell line, and 4T1 mouse breast cancer cell line. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for E-Cadherin at approximately 110 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
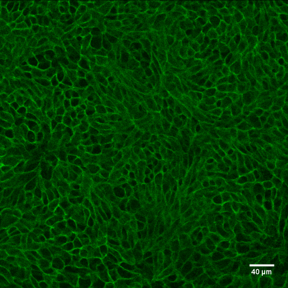
E‑Cadherin in D3 Mouse Embryonic Stem Cell Line. E-Cadherin was detected in immersion fixed D3 mouse embryonic stem cell line using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to cell surfaces. View our protocol for Fluorescent ICC Staining of Stem Cells on Coverslips.
 View Larger
View Larger
E‑Cadherin in Mouse Skin. E-Cadherin was detected in perfusion fixed frozen sections of mouse skin using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 1.7 µg/mL overnight at 4 °C. Tissue was stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to plasma membranes in keratinocytes. View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
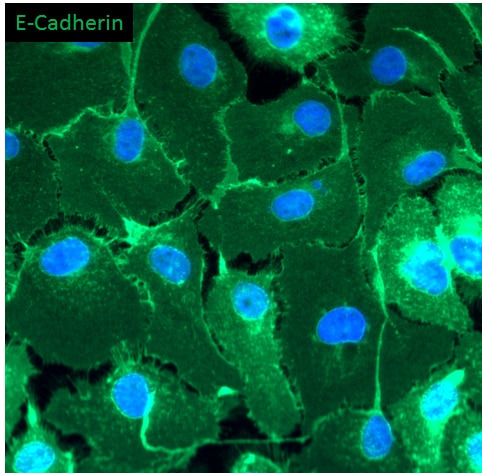
E‑Cadherin in Mouse Intestinal Organoids. E-Cadherin was detected in immersion fixed mouse intestinal organoids using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (green; Catalog # NL001) and counterstained with DAPI (blue). Magnification shown at 100X (upper panel) and 40X (lower panel). Specific staining was localized to cell surfaces. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
E‑Cadherin in Mouse Intestinal Organoids. E-Cadherin was detected in immersion fixed mouse intestinal organoids using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 493-conjugated Anti-Goat IgG Secondary Antibody (green; Catalog # NL003) and counterstained with DAPI (blue). Specific staining was localized to cell surfaces. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
E‑Cadherin in Mouse Spinal Cord. E-Cadherin was detected in perfusion fixed frozen sections of mouse spinal cord using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 1.7 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific staining was localized to dorsal horn. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of Human and Mouse E‑Cadherin by Simple WesternTM. Simple Western lane view shows lysates of 4T1 mouse breast cancer cell line, P19 mouse embryonal carcinoma cell line, A431 human epithelial carcinoma cell line, and MCF-7 human breast cancer cell line, loaded at 0.2 mg/mL. A specific band was detected for E-Cadherin at approximately 128 kDa (as indicated) using 5 µg/mL of Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Mouse E-Cadherin by Immunocytochemistry/Immunofluorescence Retention of the spermatogonial phenotype following gene correction.Immunostaining was performed on gene-corrected GT59 (left) and GT65 cells (right): DAZL, a germ cell specific marker; GFRA1, POU5F1, ETV5, CDH1, and SOHLH1, markers of undifferentiated spermatogonia. Additionally, GT59 and GT65 cells were treated with the differentiation factor, retinoic acid (1 µM) or a vehicle control and then immunostained to examine levels of ZBTB16, a marker of undifferentiated spermatogonia. Bar represents 50 microns. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25409432), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse E-Cadherin by Immunocytochemistry/Immunofluorescence Localization of MAFB in mouse testes.(A) Localization of MAFB in E18.5 mouse testes. Double immunostaining of MAFB with E-cadherin (ECAD), GATA4, or STAR is shown. Nuclei were counterstained with DAPI. The color of each marker is indicated above the images. G; germ cells. S; Sertoli cells. L; Leydig cells. MAFB was specifically detected in Leydig cells and Sertoli cells. (B) Localization of MAFB in adult mouse testes. Double immunostaining of MAFB with E-cadherin (ECAD), KIT, SCP3, PNA Lectin, or vimentin is shown. Nuclei were counterstained with DAPI. The color of each marker is indicated above the images. All seminiferous tubules shown represent stage VII. US; undifferentiated spermatogonia. DS; differentiated spermatogonia. P; pachytene spermatocytes. Sp; spermatids. S; Sertoli cells. L; Leydig cells. MAFB was specifically detected in Leydig cells, Sertoli cells, and pachytene spermatocytes. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0190800), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse E-Cadherin by Immunocytochemistry/Immunofluorescence Cellular localization of PEDF in testicular spermatogenic cells. Methanol-fixed cells were used to stain premeiotic and meiotic/postmeiotic cells as described in Figure 2. (A) alpha 6-integrin, (B) GFR-alpha, (C) CDH1, (D) and NC—IF staining without the presence of primary antibody. (E) CREM, (F) Boule, (G) Acrosin and (H) NC—IF staining without the presence of primary antibody. Scale bar: 100 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33498962), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse E-Cadherin by Immunocytochemistry/Immunofluorescence Testis morphogenesis of Mafb KO embryos developed normally.(A) Histological section of WT and KO E18.5 testes stained with HE. No morphological alteration or distribution was detectable. (B and C) Counts of Leydig and Sertoli cells from E18.5 WT and KO testes. Embryonic testes (n = 3 per genotype) were sectioned, and 4 sections for each gonad were randomly selected and stained with the germ cell marker E-cadherin (green) together with either STAR (red) or GATA4 (red). Numbers of STAR-positive cells outside seminiferous tubules (Leydig cells) or GATA4-positive cells inside the tubules (Sertoli cells) were counted per unit area. The values are the mean±S.D. *P<0.05. There was no significant difference between WT and KO cell counts. (D) The expression of genes involved in testes development and function. mRNA expression of the gene markers encoding for PGCs (Oct4), Leydig cells (Cyp17a1, StAR, Insl3, Hsd3b1, and Cyp11a1), and Sertoli cells (Amh, Sox9, and WT1) was determined by qRT-PCR in E18.5 WT and Mafb KO testes. Gene expression levels were normalized to Hprt. The bars represent the mean±SEM of five individuals. *P<0.05. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0190800), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse E-Cadherin by Immunocytochemistry/Immunofluorescence Localization of MAFB in mouse testes.(A) Localization of MAFB in E18.5 mouse testes. Double immunostaining of MAFB with E-cadherin (ECAD), GATA4, or STAR is shown. Nuclei were counterstained with DAPI. The color of each marker is indicated above the images. G; germ cells. S; Sertoli cells. L; Leydig cells. MAFB was specifically detected in Leydig cells and Sertoli cells. (B) Localization of MAFB in adult mouse testes. Double immunostaining of MAFB with E-cadherin (ECAD), KIT, SCP3, PNA Lectin, or vimentin is shown. Nuclei were counterstained with DAPI. The color of each marker is indicated above the images. All seminiferous tubules shown represent stage VII. US; undifferentiated spermatogonia. DS; differentiated spermatogonia. P; pachytene spermatocytes. Sp; spermatids. S; Sertoli cells. L; Leydig cells. MAFB was specifically detected in Leydig cells, Sertoli cells, and pachytene spermatocytes. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0190800), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse E-Cadherin by Immunocytochemistry/Immunofluorescence Testis morphogenesis of Mafb KO embryos developed normally.(A) Histological section of WT and KO E18.5 testes stained with HE. No morphological alteration or distribution was detectable. (B and C) Counts of Leydig and Sertoli cells from E18.5 WT and KO testes. Embryonic testes (n = 3 per genotype) were sectioned, and 4 sections for each gonad were randomly selected and stained with the germ cell marker E-cadherin (green) together with either STAR (red) or GATA4 (red). Numbers of STAR-positive cells outside seminiferous tubules (Leydig cells) or GATA4-positive cells inside the tubules (Sertoli cells) were counted per unit area. The values are the mean±S.D. *P<0.05. There was no significant difference between WT and KO cell counts. (D) The expression of genes involved in testes development and function. mRNA expression of the gene markers encoding for PGCs (Oct4), Leydig cells (Cyp17a1, StAR, Insl3, Hsd3b1, and Cyp11a1), and Sertoli cells (Amh, Sox9, and WT1) was determined by qRT-PCR in E18.5 WT and Mafb KO testes. Gene expression levels were normalized to Hprt. The bars represent the mean±SEM of five individuals. *P<0.05. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0190800), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of E‑Cadherin in mouse intestine. Formalin-fixed paraffin-embedded tissue sections of mouse intestine were probed for E-Cadherin mRNA (ACD RNAScope Probe, catalog # 408651; Fast Red chromogen, ACD catalog # 322360). Adjacent tissue section was processed for immunohistochemistry using goat anti-mouse E-Cadherin polyclonal antibody (R&D Systems catalog # AF748) at 0.5ug/mL with overnight incubation at 4 degrees Celsius followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog # VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to glandular cells.
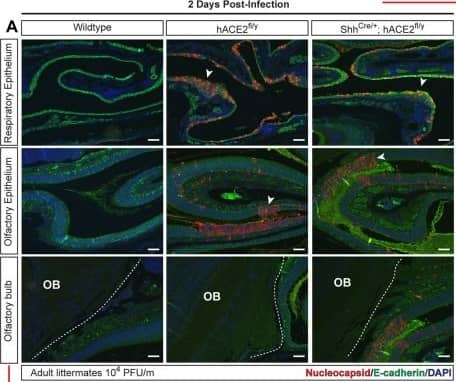 View Larger
View Larger
Detection of Mouse E-Cadherin by Immunohistochemistry-Paraffin SARS-CoV-2 infection of the OE and brain in hACE2fl mice.(A, B) Immunohistochemistry of SARS-CoV-2 nucleocapsid and epithelial cell E-cadherin in the RE, OE, and OB 2 and 5–6 days after infection of hACE2fl/y and ShhCre/+; hACE2fl/y mice. Arrowheads indicate sites of viral nucleocapsid detection. Representative of N = 4 animals per genotype and time point. Scale bars 100 μm. (C, D) Immunohistochemistry of SARS-CoV-2 nucleocapsid, neuronal NeuN, and glial cell GFAP in the cerebral cortex (Co) 2 and 5–6 days after infection. Arrowheads indicate sites of GFAP+ reactive gliosis. Arrows indicate nucleocapsid colocalization with NeuN staining. Representative of N = 4 animals per genotype and time point. Scale bars 100 μm top, 50 μm bottom. (E) Diagram of the mouse nasal cavity and cranial anatomy. (F) In situ hybridization detection of SARS-CoV-2 mRNA 5 days postinfection reveals virus in the OB and cerebral cortex of the brain, but not the OE of the nose. Scale bar 250 μm. Note: Images in each panel were taken at lower or higher magnification from the same tissue section respective to genotype and highlight different anatomical regions. OB, olfactory bulb of the brain; OE, olfactory epithelium; RE, respiratory epithelium; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36745682), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: E-Cadherin
Epithelial (E)‑Cadherin (ECAD), also known as cell-CAM120/80 in the human, uvomorulin in the mouse, Arc-1 in the dog, and L-CAM in the chicken, is a member of the cadherin family of cell adhesion molecules. Cadherins are calcium-dependent transmembrane proteins, which bind to one another in a homophilic manner. On their cytoplasmic side, they associate with the three catenins, alpha, beta, and gamma (plakoglobin). This association links the cadherin protein to the cytoskeleton. Without association with the catenins, the cadherins are non-adhesive. Cadherins play a role in development, specifically in tissue formation. They may also help to maintain tissue architecture in the adult. E-Cadherin may also play a role in tumor development, as loss of E-Cadherin has been associated with tumor invasiveness. E-Cadherin is a classical cadherin molecule. Classical cadherins consist of a large extracellular domain which contains DXD and DXNDN repeats responsible for mediating calcium‑dependent adhesion, a single-pass transmembrane domain, and a short carboxy-terminal cytoplasmic domain responsible for interacting with the catenins. E‑Cadherin contains five extracellular calcium-binding domains of approximately 110 amino acids each.
- Bussemakers, M.J.G. et al. (1993) Mol. Biol. Reports 17:123.
- Overduin, M. et al. (1995) Science 267:386.
- Takeichi, M. (1991) 251:1451.
Product Datasheets
Citations for Human/Mouse E-Cadherin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
144
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance.
Authors: Liu HX, Ermilov A, Grachtchouk M et al.
Dev Biol
-
A Fat4-Dchs1 signal between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching.
Authors: Mao Y, Francis-West P, Irvine KD.
Development.
-
Intestinal transit amplifying cells require METTL3 for growth factor signaling, KRAS expression, and cell survival
Authors: CH Danan, KE Naughton, KE Hayer, S Vellapan, EA McMillan, Y Zhou, R Matsuda, SK Nettleford, K Katada, LR Parham, X Ma, A Chowdhury, BJ Wilkins, P Shah, MD Weitzman, KE Hamilton
bioRxiv : the preprint server for biology, 2023-04-21;0(0):.
-
Effect of ectopic expression of homeoprotein EGAM1C on the cell morphology, growth, and differentiation in a mouse embryonic stem cell line, MG1.19 cells.
Authors: Iha M, Watanabe M, Kihara Y et al.
Reproduction.
-
Xenotransplanted human organoids identify transepithelial zinc transport as a key mediator of intestinal adaptation
Authors: Sampah, MES;Moore, H;Ahmad, R;Duess, J;Lu, P;Lopez, C;Steinway, S;Scheese, D;Raouf, Z;Tsuboi, K;Ding, J;Caputo, C;McFarland, M;Fulton, WB;Wang, S;Wang, M;Prindle, T;Gazit, V;Rubin, DC;Alaish, S;Sodhi, CP;Hackam, DJ;
Nature communications
Species: Human
Sample Types: Organoid
Applications: Immunohistochemistry -
Clearance of erythrocytes from the subarachnoid space through cribriform plate lymphatics in female mice
Authors: Madarasz, A;Xin, L;Proulx, ST;
EBioMedicine
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Rosiglitazone and trametinib exhibit potent anti-tumor activity in a mouse model of muscle invasive bladder cancer
Authors: Plumber, SA;Tate, T;Al-Ahmadie, H;Chen, X;Choi, W;Basar, M;Lu, C;Viny, A;Batourina, E;Li, J;Gretarsson, K;Alija, B;Molotkov, A;Wiessner, G;Lee, BHL;McKiernan, J;McConkey, DJ;Dinney, C;Czerniak, B;Mendelsohn, CL;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Critical role of thrombospondin-1 in promoting intestinal mucosal wound repair
Authors: Wilson, ZS;Raya-Sandino, A;Miranda, J;Fan, S;Brazil, JC;Quiros, M;Garcia-Hernandez, V;Liu, Q;Kim, CH;Hankenson, KD;Nusrat, A;Parkos, CA;
JCI insight
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
In vivo interaction screening reveals liver-derived constraints to metastasis
Authors: Borrelli, C;Roberts, M;Eletto, D;Hussherr, MD;Fazilaty, H;Valenta, T;Lafzi, A;Kretz, JA;Guido Vinzoni, E;Karakatsani, A;Adivarahan, S;Mannhart, A;Kimura, S;Meijs, A;Baccouche Mhamedi, F;Acar, IE;Handler, K;Ficht, X;Platt, RJ;Piscuoglio, S;Moor, AE;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Trans-omic profiling uncovers molecular controls of early human cerebral organoid formation
Authors: Chen, C;Lee, S;Zyner, KG;Fernando, M;Nemeruck, V;Wong, E;Marshall, LL;Wark, JR;Aryamanesh, N;Tam, PPL;Graham, ME;Gonzalez-Cordero, A;Yang, P;
Cell reports
Species: Human
Sample Types: Organoid
Applications: Immunohistochemistry -
Venous-plexus-associated lymphoid hubs support meningeal humoral immunity
Authors: Fitzpatrick, Z;Ghabdan Zanluqui, N;Rosenblum, JS;Tuong, ZK;Lee, CYC;Chandrashekhar, V;Negro-Demontel, ML;Stewart, AP;Posner, DA;Buckley, M;Allinson, KSJ;Mastorakos, P;Chittiboina, P;Maric, D;Donahue, D;Helmy, A;Tajsic, T;Ferdinand, JR;Portet, A;Peñalver, A;Gillman, E;Zhuang, Z;Clatworthy, MR;McGavern, DB;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Measurement of adhesion and traction of cells at high yield (MATCHY) reveals an energetic ratchet driving nephron condensation
Authors: Liu, J;Prahl, LS;Huang, A;Hughes, AJ;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Intestinal transit-amplifying cells require METTL3 for growth factor signaling and cell survival
Authors: Danan, CH;Naughton, KE;Hayer, KE;Vellappan, S;McMillan, EA;Zhou, Y;Matsuda, R;Nettleford, SK;Katada, K;Parham, LR;Ma, X;Chowdhury, A;Wilkins, BJ;Shah, P;Weitzman, MD;Hamilton, KE;
JCI insight
Species: Transgenic Mouse
Sample Types: Organoid
Applications: Immunohistochemistry -
Transcriptomic analysis reveals partial epithelial-mesenchymal transition and inflammation as common pathogenic mechanisms in hypertensive nephrosclerosis and Type 2 diabetic nephropathy
Authors: Nordbø, OP;Landolt, L;Eikrem, Ø;Scherer, A;Leh, S;Furriol, J;Apeland, T;Mydel, P;Marti, HP;
Physiological reports
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Spatial-temporal proliferation of hepatocytes during pregnancy revealed by genetic lineage tracing
Authors: He, S;Guo, Z;Zhou, M;Wang, H;Zhang, Z;Shi, M;Li, X;Yang, X;He, L;
Cell stem cell
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
VE-cadherin in arachnoid and pia mater cells serves as a suitable landmark for in vivo imaging of CNS immune surveillance and inflammation
Authors: Mapunda JA, Pareja J, Vladymyrov M et al.
Nat Commun
-
Coordination between ECM and cell-cell adhesion regulates the development of islet aggregation, architecture, and functional maturation
Authors: Tixi W, Maldonado M, Chang YT et al.
eLife
-
An inducible hACE2 transgenic mouse model recapitulates SARS-CoV-2 infection and pathogenesis in vivo
Authors: Liu, K;Tang, M;Xu, W;Meng, X;Jin, H;Han, M;Pu, J;Li, Y;Jiao, F;Sun, R;Shen, R;Lui, KO;Lu, L;Zhou, B;
Proceedings of the National Academy of Sciences of the United States of America
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Bacterial meningitis in the early postnatal mouse studied at single-cell resolution
Authors: Wang J, Rattner A, Nathans J
eLife
-
Genetic recording of in vivo cell proliferation by ProTracer
Authors: Liu, X;Weng, W;He, L;Zhou, B;
Nature protocols
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neural crest E-cadherin loss drives cleft lip/palate by epigenetic modulation via pro-inflammatory gene-environment interaction
Authors: Alvizi, L;Nani, D;Brito, LA;Kobayashi, GS;Passos-Bueno, MR;Mayor, R;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Opposing roles for TGF?- and BMP-signaling during nascent alveolar differentiation in the developing human lung
Authors: Frum, T;Hsu, PP;Hein, RFC;Conchola, AS;Zhang, CJ;Utter, OR;Anand, A;Zhang, Y;Clark, SG;Glass, I;Sexton, JZ;Spence, JR;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Organoids
Applications: IHC -
IGFBP2 expressing midlobular hepatocytes preferentially contribute to liver homeostasis and regeneration
Authors: Lin, YH;Wei, Y;Zeng, Q;Wang, Y;Pagani, CA;Li, L;Zhu, M;Wang, Z;Hsieh, MH;Corbitt, N;Zhang, Y;Sharma, T;Wang, T;Zhu, H;
Cell stem cell
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Role of enterocyte Enpp2 and autotaxin in regulating lipopolysaccharide levels, systemic inflammation, and atherosclerosis
Authors: Arnab Chattopadhyay, Pallavi Mukherjee, Dawoud Sulaiman, Huan Wang, Victor Girjalva, Nasrin Dorreh et al.
Journal of Lipid Research
-
Non-optimal bacteria species induce neutrophil-driven inflammation and barrier disruption in the female genital tract
Authors: M Costa Fuji, A Yazdanpana, S Horne, A Lamont, P Lopez, C Farr Zuend, K Birse, M Taverner, R Greenslade, M Abou, L Noel-Romas, B Abrenica, O Ajibola, N Ikeogu, RC Su, LR McKinnon, H Pymar, V Poliquin, AR Berard, A Burgener, TT Murooka
Mucosal Immunology, 2023-04-29;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves
Authors: I Spera, N Cousin, M Ries, A Kedracka, A Castillo, S Aleandri, M Vladymyrov, JA Mapunda, B Engelhardt, P Luciani, M Detmar, ST Proulx
EBioMedicine, 2023-04-10;91(0):104558.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Uropathogenic Escherichia coli infection-induced epithelial trained immunity impacts urinary tract disease outcome
Authors: SK Russell, JK Harrison, BS Olson, HJ Lee, VP O'Brien, X Xing, J Livny, L Yu, EDO Roberson, R Bomjan, C Fan, M Sha, S Estfanous, AO Amer, M Colonna, TS Stappenbec, T Wang, TJ Hannan, SJ Hultgren
Nature Microbiology, 2023-04-10;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
PTPN2 Is a Critical Regulator of Ileal Paneth Cell Viability and Function in Mice
Authors: Vinicius Canale, Marianne R. Spalinger, Rocio Alvarez, Anica Sayoc-Becerra, Golshid Sanati, Salomon Manz et al.
Cellular and Molecular Gastroenterology and Hepatology
-
Taste papilla cell differentiation requires tongue mesenchyme via ALK3-BMP signaling to regulate the production of secretory proteins
Authors: M Ishan, Z Wang, P Zhao, Y Yao, S Stice, L Wells, Y Mishina, HX Liu
bioRxiv : the preprint server for biology, 2023-04-04;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
EPIREGULIN creates a developmental niche for spatially organized human intestinal enteroids
Authors: Charlie J. Childs, Emily M. Holloway, Caden W. Sweet, Yu-Hwai Tsai, Angeline Wu, Abigail Vallie et al.
JCI Insight
-
Non-canonical functions of SNAIL drive context-specific cancer progression
Authors: MC Paul, C Schneeweis, C Falcomatà, C Shan, D Rossmeisl, S Koutsouli, C Klement, M Zukowska, SA Widholz, M Jesinghaus, KK Heuermann, T Engleitner, B Seidler, K Sleiman, K Steiger, M Tschurtsch, B Walter, SA Weidemann, R Pietsch, A Schnieke, RM Schmid, MS Robles, G Andrieux, M Boerries, R Rad, G Schneider, D Saur
Nature Communications, 2023-03-07;14(1):1201.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Bone metastasis initiation is coupled with bone remodeling through osteogenic differentiation of NG2+ cells
Authors: W Zhang, Z Xu, X Hao, T He, J Li, Y Shen, K Liu, Y Gao, J Liu, D Edwards, AM Muscarella, L Wu, L Yu, L Xu, X Chen, YH Wu, IL Bado, Y Ding, S Aguirre, H Wang, Z Gugala, RL Satcher, ST Wong, XH Zhang
Cancer Discovery, 2023-02-06;0(0):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cell-autonomous requirement for ACE2 across organs in lethal mouse SARS-CoV-2 infection
Authors: AT Tang, DW Buchholz, KM Szigety, B Imbiakha, S Gao, M Frankfurte, M Wang, J Yang, P Hewins, P Mericko-Is, NA Leu, S Sterling, IA Monreal, J Sahler, A August, X Zhu, KA Jurado, M Xu, EE Morrisey, SE Millar, HC Aguilar, ML Kahn
PloS Biology, 2023-02-06;21(2):e3001989.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Arginase-1 inhibition reduces migration ability and metastatic colonization of colon cancer cells
Authors: Xiangdong Wang, Huihui Xiang, Yujiro Toyoshima, Weidong Shen, Shunsuke Shichi, Hiroki Nakamoto et al.
Cancer & Metabolism
-
Non-canonical functions of SNAIL drive context-specific cancer progression
Authors: MC Paul, C Schneeweis, C Falcomatà, C Shan, D Rossmeisl, S Koutsouli, C Klement, M Zukowska, SA Widholz, M Jesinghaus, KK Heuermann, T Engleitner, B Seidler, K Sleiman, K Steiger, M Tschurtsch, B Walter, SA Weidemann, R Pietsch, A Schnieke, RM Schmid, MS Robles, G Andrieux, M Boerries, R Rad, G Schneider, D Saur
Nature Communications, 2023;14(1):1201.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Multiscale profiling of protease activity in cancer
Authors: AP Amini, JD Kirkpatric, CS Wang, AM Jaeger, S Su, S Naranjo, Q Zhong, CM Cabana, T Jacks, SN Bhatia
Nature Communications, 2022-10-03;13(1):5745.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The NALCN channel regulates metastasis and nonmalignant cell dissemination
Authors: EP Rahrmann, D Shorthouse, A Jassim, LP Hu, M Ortiz, B Mahler-Ara, P Vogel, M Paez-Ribes, A Fatemi, GJ Hannon, R Iyer, JA Blundon, FC Lourenço, J Kay, RM Nazarian, BA Hall, SS Zakharenko, DJ Winton, L Zhu, RJ Gilbertson
Nature Genetics, 2022-09-29;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Focal adhesion kinase-mediated signaling controls the onset of pancreatic cell differentiation
Authors: Uylissa A. Rodriguez, Shakti Dahiya, Michelle L. Raymond, Chenxi Gao, Christina P. Martins-Cargill, Jon D. Piganelli et al.
Development
-
Adherens junctions stimulate and spatially guide integrin activation and extracellular matrix deposition
Authors: R Hadjisavva, O Anastasiou, PS Ioannou, M Zheltkova, PA Skourides
Oncogene, 2022-07-19;40(3):111091.
Species: Xenopus
Sample Types: Embryos
Applications: IHC -
Lymphatics act as a signaling hub to regulate intestinal stem cell activity
Authors: RE Niec, T Chu, M Schernthan, S Gur-Cohen, L Hidalgo, HA Pasolli, KA Luckett, Z Wang, SR Bhalla, F Cambuli, RP Kataru, K Ganesh, BJ Mehrara, D Pe'er, E Fuchs
Cell Stem Cell, 2022-06-20;29(7):1067-1082.e18.
Species: Human, Mouse
Sample Types: Cell Lysates, Tissue Homogenates
Applications: Western Blot -
Ex Vivo Perfusion Using a Mathematical Modeled, Controlled Gas Exchange Self-Contained Bioreactor Can Maintain a Mouse Kidney for Seven Days
Authors: N Won, J Castillo-P, X Tan, J Ford, D Heath, LI Mazilescu, M Selzner, IM Rogers
Cells, 2022-06-02;11(11):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dual Cre and Dre recombinases mediate synchronized lineage tracing and cell subset ablation in vivo
Authors: Haixiao Wang, Lingjuan He, Yan Li, Wenjuan Pu, Shaohua Zhang, Ximeng Han et al.
Journal of Biological Chemistry
-
A myosin chaperone, UNC-45A, is a novel regulator of intestinal epithelial barrier integrity and repair.
Authors: Susana L, Alexander C, Afshin K et al.
FASEB J.
-
Cyclic GMP‐AMP synthase contributes to epithelial homeostasis in intestinal inflammation via Beclin‐1‐mediated autophagy
Authors: Sidrah Khan, Heather L. Mentrup, Elizabeth A. Novak, Vei Shaun Siow, Qian Wang, Erin C. Crawford et al.
The FASEB Journal
-
Amniogenesis occurs in two independent waves in primates
Authors: M Rostovskay, S Andrews, W Reik, PJ Rugg-Gunn
Cell Stem Cell, 2022-04-18;29(5):744-759.e6.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Loss of Rnf31 and Vps4b sensitizes pancreatic cancer to T cell-mediated killing
Authors: N Frey, L Tortola, D Egli, S Janjuha, T Rothgangl, KF Marquart, F Ampenberge, M Kopf, G Schwank
Nature Communications, 2022-04-04;13(1):1804.
Species: Human
Sample Types: Organoids
Applications: IHC -
Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate
Authors: Martin Hsu, Collin Laaker, Andy Madrid, Melinda Herbath, Yun Hwa Choi, Matyas Sandor et al.
Nature Immunology
-
Generation of human tonsil epithelial organoids as an ex vivo model for SARS-CoV-2 infection
Authors: Han Kyung Kim, Hyeryeon Kim, Myoung Kyu Lee, Woo Hee Choi, Yejin Jang, Jin Soo Shin et al.
Biomaterials
-
Suspension culture promotes serosal mesothelial development in human intestinal organoids
Authors: MM Capeling, S Huang, CJ Childs, JH Wu, YH Tsai, A Wu, N Garg, EM Holloway, N Sundaram, C Bouffi, M Helmrath, JR Spence
Cell Reports, 2022-02-15;38(7):110379.
Species: Human
Sample Types: Oraganoids
Applications: IHC -
Fundamentals of vaping-associated pulmonary injury leading to severe respiratory distress
Authors: Carolina Esquer, Oscar Echeagaray, Fareheh Firouzi, Clarissa Savko, Grant Shain, Pria Bose et al.
Life Science Alliance
-
Macrophage COX2 Mediates Efferocytosis, Resolution Reprogramming, and Intestinal Epithelial Repair
Authors: David Meriwether, Anthony E. Jones, Julianne W. Ashby, R. Sergio Solorzano-Vargas, Nasrin Dorreh, Shoreh Noori et al.
Cellular and Molecular Gastroenterology and Hepatology
-
Oxidized phospholipids cause changes in jejunum mucus that induce dysbiosis and systemic inflammation
Authors: Pallavi Mukherjee, Arnab Chattopadhyay, Victor Grijalva, Nasrin Dorreh, Venu Lagishetty, Jonathan P. Jacobs et al.
Journal of Lipid Research
-
E-Cadherin-Deficient Epithelial Cells Are Sensitive to HDAC Inhibitors
Authors: L Decourtye-, N Bougen-Zhu, T Godwin, T Brew, E Schulpen, MA Black, P Guilford
Cancers, 2021-12-30;14(1):.
Species: Mouse
Sample Types: Organoids
Applications: IHC, Western Blot -
Loss of E-Cadherin Leads to Druggable Vulnerabilities in Sphingolipid Metabolism and Vesicle Trafficking
Authors: T Brew, N Bougen-Zhu, W Mitchell, L Decourtye, E Schulpen, Y Nouri, T Godwin, P Guilford
Cancers, 2021-12-26;14(1):.
Species: Transgenic Mouse
Sample Types: Organoids
Applications: IHC -
Deletion of Nf2 in neural crest‐derived tongue mesenchyme alters tongue shape and size, Hippo signalling and cell proliferation in a region‐ and stage‐specific manner
Authors: Mohamed Ishan, Guiqian Chen, Wenxin Yu, Zhonghou Wang, Marco Giovannini, Xinwei Cao et al.
Cell Proliferation
-
Differentiation of mouse fetal lung alveolar progenitors in serum-free organotypic cultures
Authors: K Gkatzis, P Panza, S Peruzzo, DY Stainier
Elife, 2021-09-29;10(0):.
Species: Mouse
Sample Types: Organoids
Applications: IHC -
Signaling Modulation by miRNA-221-3p During Tooth Morphogenesis in Mice
Authors: Yam Prasad Aryal, Tae-Young Kim, Eui-Seon Lee, Chang-Hyeon An, Ji-Youn Kim, Hitoshi Yamamoto et al.
Frontiers in Cell and Developmental Biology
-
Reciprocal interplay between asporin and decorin: Implications in gastric cancer prognosis
Authors: D Basak, Z Jamal, A Ghosh, PK Mondal, P Dey Talukd, S Ghosh, B Ghosh Roy, R Ghosh, A Halder, A Chowdhury, GK Dhali, BK Chattopadh, ML Saha, A Basu, S Roy, C Mukherjee, NK Biswas, U Chatterji, S Datta
PLoS ONE, 2021-08-11;16(8):e0255915.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
M1 Macrophage-Derived Nanovesicles Repolarize M2 Macrophages for Inhibiting the Development of Endometriosis
Authors: Qiuju Li, Ming Yuan, Xue Jiao, Yufei Huang, Jing Li, Dong Li et al.
Frontiers in Immunology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19
Authors: Ji Hoon Ahn, JungMo Kim, Seon Pyo Hong, Sung Yong Choi, Myung Jin Yang, Young Seok Ju et al.
Journal of Clinical Investigation
-
Charting human development using a multi-endodermal organ atlas and organoid models
Authors: Qianhui Yu, Umut Kilik, Emily M. Holloway, Yu-Hwai Tsai, Christoph Harmel, Angeline Wu et al.
Cell
-
Microtissue Geometry and Cell‐Generated Forces Drive Patterning of Liver Progenitor Cell Differentiation in 3D
Authors: Ian C. Berg, Erfan Mohagheghian, Krista Habing, Ning Wang, Gregory H. Underhill
Advanced Healthcare Materials
-
Homophilic and heterophilic cadherin bond rupture forces in homo- or hetero-cellular systems measured by AFM-based single-cell force spectroscopy
Authors: Prem Kumar Viji Babu, Ursula Mirastschijski, Ganzanfer Belge, Manfred Radmacher
European Biophysics Journal
-
In vitro models of fetal lung development to enhance research into congenital lung diseases
Authors: Soichi Shibuya, Jessica Allen-Hyttinen, Paolo De Coppi, Federica Michielin
Pediatric Surgery International
-
Post-transcriptional repression of circadian component CLOCK regulates cancer-stemness in murine breast cancer cells
Authors: Takashi Ogino, Naoya Matsunaga, Takahiro Tanaka, Tomohito Tanihara, Hideki Terajima, Hikari Yoshitane et al.
eLife
-
Primary cilia-dependent lipid raft/caveolin dynamics regulate adipogenesis
Authors: D Yamakawa, D Katoh, K Kasahara, T Shiromizu, M Matsuyama, C Matsuda, Y Maeno, M Watanabe, Y Nishimura, M Inagaki
Cell Reports, 2021-03-09;34(10):108817.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Paneth cell–derived growth factors support tumorigenesis in the small intestine
Authors: Qing Chen, Kohei Suzuki, Luis Sifuentes-Dominguez, Naoteru Miyata, Jie Song, Adam Lopez et al.
Life Science Alliance
-
The Presence of Colony-Stimulating Factor-1 and Its Receptor in Different Cells of the Testis; It Involved in the Development of Spermatogenesis In Vitro
Authors: Sawaied A, Arazi E, AbuElhija A et al.
International Journal of Molecular Sciences
-
Maternal aryl hydrocarbon receptor activation protects newborns against necrotizing enterocolitis
Authors: P Lu, Y Yamaguchi, WB Fulton, S Wang, Q Zhou, H Jia, ML Kovler, AG Salazar, M Sampah, T Prindle, P Wipf, CP Sodhi, DJ Hackam
Nature Communications, 2021-02-15;12(1):1042.
Species: Human, Mouse, Porcine
Sample Types: Whole Tissue
Applications: IHC -
The Expression Levels and Cellular Localization of Pigment Epithelium Derived Factor (PEDF) in Mouse Testis: Its Possible Involvement in the Differentiation of Spermatogonial Cells
Authors: N Bagdadi, A Sawaied, A AbuMadighe, E Lunenfeld, M Huleihel
International Journal of Molecular Sciences, 2021-01-24;22(3):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mapping Development of the Human Intestinal Niche at Single-Cell Resolution
Authors: EM Holloway, M Czerwinski, YH Tsai, JH Wu, A Wu, CJ Childs, KD Walton, CW Sweet, Q Yu, I Glass, B Treutlein, JG Camp, JR Spence
Cell Stem Cell, 2020-12-04;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Tim-3 promotes cell aggressiveness and paclitaxel resistance through NF-kappa B/STAT3 signalling pathway in breast cancer cells
Authors: Yizi Cong, Department of Breast Surgery, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan 250033, China, Yuxin Cui, Shiguang Zhu, Jianqiao Cao, Haidong Zou et al.
Chinese Journal of Cancer Research
-
Developmental Roles of FUSE Binding Protein 1 (Fubp1) in Tooth Morphogenesis
Authors: Yam Prasad Aryal, Sanjiv Neupane, Tae-Young Kim, Eui-Seon Lee, Nitin Kumar Pokhrel, Chang-Yeol Yeon et al.
International Journal of Molecular Sciences
-
Three-Dimensional Human Alveolar Stem Cell Culture Models Reveal Infection Response to SARS-CoV-2
Authors: J Youk, T Kim, KV Evans, YI Jeong, Y Hur, SP Hong, JH Kim, K Yi, SY Kim, KJ Na, T Bleazard, HM Kim, M Fellows, KT Mahbubani, K Saeb-Parsy, SY Kim, YT Kim, GY Koh, BS Choi, YS Ju, JH Lee
Cell Stem Cell, 2020-10-21;27(6):905-919.e10.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
RUNX1 marks a luminal castration-resistant lineage established at the onset of prostate development
Authors: Renaud Mevel, Ivana Steiner, Susan Mason, Laura CA Galbraith, Rahima Patel, Muhammad ZH Fadlullah et al.
eLife
-
Mycotoxin Deoxynivalenol Has Different Impacts on Intestinal Barrier and Stem Cells by Its Route of Exposure
Authors: H Hanyu, Y Yokoi, K Nakamura, T Ayabe, K Tanaka, K Uno, K Miyajima, Y Saito, K Iwatsuki, M Shimizu, M Tadaishi, K Kobayashi-
Toxins (Basel), 2020-09-24;12(10):.
Species: Mouse
Sample Types: Organoid
Applications: IHC -
Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth
Authors: Krivanek J, Soldatov RA, Kastriti ME et al.
Nature Communications
-
Calcium-Binding Protein S100P Promotes Tumor Progression but Enhances Chemosensitivity in Breast Cancer
Authors: Yizi Cong, Yuxin Cui, Suxia Wang, Lei Jiang, Jianqiao Cao, Shiguang Zhu et al.
Frontiers in Oncology
-
Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi
Authors: SP Hong, MJ Yang, H Cho, I Park, H Bae, K Choe, SH Suh, RH Adams, K Alitalo, D Lim, GY Koh
Nat Commun, 2020-08-14;11(1):4102.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Three-Dimensional Imaging and Gene Expression Analysis Upon Enzymatic Isolation of the Tongue Epithelium
Authors: Christian T. Meisel, Pierfrancesco Pagella, Cristina Porcheri, Thimios A. Mitsiadis
Frontiers in Physiology
-
Dysregulation of intestinal epithelial CFTR-dependent Cl- ion transport and paracellular barrier function drives gastrointestinal symptoms of food-induced anaphylaxis in mice
Authors: A Yamani, D Wu, R Ahrens, L Waggoner, TK Noah, V Garcia-Her, C Ptaschinsk, CA Parkos, NW Lukacs, A Nusrat, SP Hogan
Mucosal Immunol, 2020-06-23;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Genetic and structural analysis of the in vivo functional redundancy between murine NANOS2 and NANOS3
Authors: Danelle Wright, Makoto Kiso, Yumiko Saga
Development
-
CD137 Signaling Regulates Acute Colitis via RALDH2-Expressing CD11b-CD103+ DCs
Authors: J Jin, IH Jung, SH Moon, S Jeon, SJ Jeong, SK Sonn, S Seo, MN Lee, EJ Song, HY Kweon, S Kim, TK Kim, J Kim, HR Cho, JH Choi, B Kwon, GT Oh
Cell Rep, 2020-03-24;30(12):4124-4136.e5.
Species: Mouse
Sample Types: Colon Tissue
Applications: IHC -
Desmocollin-2 promotes intestinal mucosal repair by controlling integrin-dependent cell adhesion and migration
Authors: Sven Flemming, Anny-Claude Luissint, Dennis H. M. Kusters, Arturo Raya-Sandino, Shuling Fan, Dennis W. Zhou et al.
Molecular Biology of the Cell
-
Genome-Scale CRISPR Screening in Human Intestinal Organoids Identifies Drivers of TGF-&beta Resistance
Authors: T Ringel, N Frey, F Ringnalda, S Janjuha, S Cherkaoui, S Butz, S Srivatsa, M Pirkl, G Russo, L Villiger, G Rogler, H Clevers, N Beerenwink, N Zamboni, T Baubec, G Schwank
Cell Stem Cell, 2020-03-05;26(3):431-440.e8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Downregulation of SETD7 promotes migration and invasion of lung cancer cells via JAK2/STAT3 pathway
Authors: Limin Cao, Yinghui Ren, Xueru Guo, Limin Wang, Qicheng Zhang, Xueqin Li et al.
International Journal of Molecular Medicine
-
Disruption of the nectin-afadin complex recapitulates features of the human cleft lip/palate syndrome CLPED1
Authors: Kendall J. Lough, Danielle C. Spitzer, Abby J. Bergman, Jessica J. Wu, Kevin M. Byrd, Scott E. Williams
Development
-
Increased activity of mesenchymal ALK2‐BMP signaling causes posteriorly truncated microglossia and disorganization of lingual tissues
Authors: Mohamed Ishan, Guiqian Chen, Chenming Sun, Shi‐You Chen, Yoshihiro Komatsu, Yuji Mishina et al.
genesis
-
A novel role for necroptosis in the pathogenesis of necrotizing enterocolitis
Authors: AD Werts, WB Fulton, MR Ladd, A Saad-Eldin, YX Chen, ML Kovler, H Jia, EC Banfield, R Buck, K Goerhing, T Prindle, S Wang, Q Zhou, P Lu, Y Yamaguchi, CP Sodhi, DJ Hackam
Cell Mol Gastroenterol Hepatol, 2019-11-19;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lack of whey acidic protein four disulphide core (WFDC) 2 protease inhibitor causes neonatal death from respiratory failure in mice
Authors: K Nakajima, M Ono, U Radovi?, S Dizdarevi?, SI Tomizawa, K Kuroha, G Naganatsu, I Hoshi, R Matsunaga, T Shirakawa, T Kurosawa, Y Miyazaki, M Seki, Y Suzuki, H Koseki, M Nakamura, T Suda, K Ohbo
Dis Model Mech, 2019-11-12;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Epithelial CD47 is critical for mucosal repair in the murine intestine in vivo
Authors: M Reed, AC Luissint, V Azcutia, S Fan, MN O'Leary, M Quiros, J Brazil, A Nusrat, CA Parkos
Nat Commun, 2019-11-01;10(1):5004.
Species: Mouse
Sample Types: Cells
Applications: IF -
Pparg promotes differentiation and regulates mitochondrial gene expression in bladder epithelial cells
Authors: C Liu, T Tate, E Batourina, ST Truschel, S Potter, M Adam, T Xiang, M Picard, M Reiley, K Schneider, M Tamargo, C Lu, X Chen, J He, H Kim, CL Mendelsohn
Nat Commun, 2019-10-09;10(1):4589.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Pharmacological reversal of renal cysts from secretion to absorption suggests a potential therapeutic strategy for managing polycystic kidney disease
Authors: MK Yanda, B Cha, C Cebotaru, L Cebotaru
J. Biol. Chem., 2019-09-30;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: Confocal Microscopy -
Disruption of Robo2-Baiap2 integrated signaling drives cystic disease
Authors: Qinggang Li, Shaoyuan Cui, Qian Ma, Ying Liu, Hongyu Yu, GuangRui Geng et al.
JCI Insight
-
Early taste buds are from Shh+ epithelial cells of tongue primordium in distinction from mature taste bud cells which arise from surrounding tissue compartments
Authors: Naomi Kramer, Guiqian Chen, Mohamed Ishan, Xiaogang Cui, Hong-Xiang Liu
Biochemical and Biophysical Research Communications
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Loss of BCL9/9l suppresses Wnt driven tumourigenesis in models that recapitulate human cancer
Authors: DM Gay, RA Ridgway, M Müeller, MC Hodder, A Hedley, W Clark, JD Leach, R Jackstadt, C Nixon, DJ Huels, AD Campbell, TG Bird, OJ Sansom
Nat Commun, 2019-02-13;10(1):723.
Species: Mouse
Sample Types: Whole Tissue
Applications: Proximity Ligation Assay (PLA) -
Pancreatic Cell Fate Determination Relies on Notch Ligand Trafficking by NFIA
Authors: MA Scavuzzo, J Chmielowie, D Yang, K Wamble, LS Chaboub, L Duraine, B Tepe, SM Glasgow, BR Arenkiel, C Brou, B Deneen, M Borowiak
Cell Rep, 2018-12-26;25(13):3811-3827.e7.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Host restriction of Escherichia coli recurrent urinary tract infection occurs in a bacterial strain-specific manner
Authors: VP O'Brien, DA Dorsey, TJ Hannan, SJ Hultgren
PLoS Pathog., 2018-12-13;14(12):e1007457.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
A novel role for C–C motif chemokine receptor 2 during infection with hypervirulent Mycobacterium tuberculosis
Authors: Micah D. Dunlap, Nicole Howard, Shibali Das, Ninecia Scott, Mushtaq Ahmed, Oliver Prince et al.
Mucosal Immunology
-
Analysis of leukocyte transepithelial migration using an in vivo murine colonic loop model
Authors: S Flemming, AC Luissint, A Nusrat, CA Parkos
JCI Insight, 2018-10-18;3(20):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Polyploid Superficial Cells that Maintain the Urothelial Barrier Are Produced via Incomplete Cytokinesis and Endoreplication
Authors: J Wang, E Batourina, K Schneider, S Souza, T Swayne, C Liu, CD George, T Tate, H Dan, G Wiessner, Y Zhuravlev, JC Canman, IU Mysorekar, CL Mendelsohn
Cell Rep, 2018-10-09;25(2):464-477.e4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Activated natural killer T cells in mice induce acute kidney injury with hematuria through possibly common mechanisms shared by human CD56+ T cells
Authors: Takahiro Uchida, Hiroyuki Nakashima, Seigo Ito, Takuya Ishikiriyama, Masahiro Nakashima, Shuhji Seki et al.
American Journal of Physiology-Renal Physiology
-
Liver regeneration requires Yap1-TGF beta -dependent epithelial-mesenchymal transition in hepatocytes
Authors: Seh-Hoon Oh, Marzena Swiderska-Syn, Mark L. Jewell, Richard T. Premont, Anna Mae Diehl
Journal of Hepatology
-
Role for the transcriptional activator ZRF1 in early metastatic events in breast cancer progression and endocrine resistance
Authors: Aysegül Kaymak, Sergi Sayols, Thaleia Papadopoulou, Holger Richly
Oncotarget
-
GILZ-dependent modulation of mTORC1 regulates spermatogonial maintenance
Authors: Hue M. La, Ai-Leen Chan, Julien M. D. Legrand, Fernando J. Rossello, Christina G. Gangemi, Antonella Papa et al.
Development
-
Desmosomal cadherin association with Tctex-1 and cortactin-Arp2/3 drives perijunctional actin polymerization to promote keratinocyte delamination
Authors: O Nekrasova, RM Harmon, JA Broussard, JL Koetsier, LM Godsel, GN Fitz, ML Gardel, KJ Green
Nat Commun, 2018-03-13;9(1):1053.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Reciprocal Spatiotemporally Controlled Apoptosis Regulates Wolffian Duct Cloaca Fusion
Authors: Masato Hoshi, Antoine Reginensi, Matthew S. Joens, James A. J. Fitzpatrick, Helen McNeill, Sanjay Jain
Journal of the American Society of Nephrology
-
NANOS2 acts as an intrinsic regulator of gonocytes-to-spermatogonia transition in the murine testes
Authors: HP Pui, Y Saga
Mech. Dev., 2018-01-12;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Combined mutation of Apc, Kras and Tgfbr2 effectively drives metastasis of intestinal cancer
Authors: E Sakai, M Nakayama, H Oshima, Y Kouyama, A Niida, S Fujii, A Ochiai, KI Nakayama, K Mimori, Y Suzuki, CP Hong, CY Ock, SJ Kim, M Oshima
Cancer Res., 2017-12-27;0(0):.
-
Multicolor quantitative confocal imaging cytometry
Authors: DL Coutu, KD Kokkaliari, L Kunz, T Schroeder
Nat. Methods, 2017-11-13;15(1):39-46.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic ? Cell Hyperplasia in Mice
Authors: J Kim, H Okamoto, Z Huang, G Anguiano, S Chen, Q Liu, K Cavino, Y Xin, E Na, R Hamid, J Lee, B Zambrowicz, R Unger, AJ Murphy, Y Xu, GD Yancopoulo, WH Li, J Gromada
Cell Metab., 2017-06-06;25(6):1348-1361.e8.
-
Distinct Requirements for FGFR1 and FGFR2 in Primitive Endoderm Development and Exit from Pluripotency
Authors: A Molotkov, P Mazot, JR Brewer, RM Cinalli, P Soriano
Dev. Cell, 2017-05-25;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection
Authors: P Treerat, O Prince, A Cruz-Lagun, M Muñoz-Torr, MA Salazar-Le, M Selman, B Fallert-Ju, TA Reinhardt, JF Alcorn, D Kaushal, J Zuñiga, J Rangel-Mor, JK Kolls, SA Khader
Mucosal Immunol, 2017-03-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Maintenance of Taste Organs Is Strictly Dependent on Epithelial Hedgehog/GLI Signaling
PLoS Genet, 2016-11-28;12(11):e1006442.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease
Nat Microbiol, 2016-10-31;2(0):16196.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress
Authors: Wakana Ohashi, Shunsuke Kimura, Toshihiko Iwanaga, Yukihiro Furusawa, Tarou Irié, Hironori Izumi et al.
PLOS Genetics
-
Western diet enhances benzo(a)pyrene-induced colon tumorigenesis in a polyposis in rat coli (PIRC) rat model of colon cancer
Authors: Kelly L. Harris, Stephanie R. Pulliam, Emmanuel Okoro, Zhongmao Guo, Mary K. Washington, Samuel E. Adunyah et al.
Oncotarget
-
Dazl is a target RNA suppressed by mammalian NANOS2 in sexually differentiating male germ cells
Authors: Y Kato, T Katsuki, H Kokubo, A Masuda, Y Saga
Nat Commun, 2016-04-13;7(0):11272.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
E-cadherin can limit the transforming properties of activating beta-catenin mutations.
Authors: Huels D, Ridgway R, Radulescu S, Leushacke M, Campbell A, Biswas S, Leedham S, Serra S, Chetty R, Moreaux G, Parry L, Matthews J, Song F, Hedley A, Kalna G, Ceteci F, Reed K, Meniel V, Maguire A, Doyle B, Soderberg O, Barker N, Watson A, Larue L, Clarke A, Sansom O
EMBO J, 2015-08-03;34(18):2321-33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Yap and Taz are required for Ret-dependent urinary tract morphogenesis
Authors: Antoine Reginensi, Masato Hoshi, Sami Kamel Boualia, Maxime Bouchard, Sanjay Jain, Helen McNeill
Development
-
Wnt5a and Wnt11 regulate mammalian anterior-posterior axis elongation
Authors: Philipp Andre, Hai Song, Wantae Kim, Andreas Kispert, Yingzi Yang
Development
-
Genome Editing in Mouse Spermatogonial Stem/Progenitor Cells Using Engineered Nucleases
Authors: Danielle A. Fanslow, Stacey E. Wirt, Jenny C. Barker, Jon P. Connelly, Matthew H. Porteus, Christina Tenenhaus Dann
PLoS ONE
-
Mitogen-activated protein kinase (MAPK) pathway regulates branching by remodeling epithelial cell adhesion.
Authors: Ihermann-Hella A, Lume M, Miinalainen I, Pirttiniemi A, Gui Y, Peranen J, Charron J, Saarma M, Costantini F, Kuure S
PLoS Genet, 2014-03-06;10(3):e1004193.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
AKT3 regulates ErbB2, ErbB3 and estrogen receptor alpha expression and contributes to endocrine therapy resistance of ErbB2(+) breast tumor cells from Balb-neuT mice.
Authors: Grabinski N, Mollmann K, Milde-Langosch K, Muller V, Schumacher U, Brandt B, Pantel K, Jucker M
Cell Signal, 2014-01-24;26(5):1021-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Annexin A2 mediates secretion of collagen VI, pulmonary elasticity and apoptosis of bronchial epithelial cells.
Authors: Dassah M, Almeida D, Hahn R, Bonaldo P, Worgall S, Hajjar K
J Cell Sci, 2013-12-19;127(0):828-44.
Species: Mouse
Sample Types: Whole Tissue
Applications: Neutralization -
Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules.
Authors: Miyauchi, Eiji, Ogita, Tasuku, Miyamoto, Junki, Kawamoto, Seiji, Morita, Hidetosh, Ohno, Hiroshi, Suzuki, Takuya, Tanabe, Soichi
PLoS ONE, 2013-11-08;8(11):e79735.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
MMP20 modulates cadherin expression in ameloblasts as enamel develops.
Authors: Guan X, Bartlett J
2013-09-25;92(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury.
Authors: Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield J
J Am Soc Nephrol, 2013-03-14;24(4):559-72.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis.
Authors: Neal M, Sodhi C, Dyer M, Craig B, Good M, Jia H, Yazji I, Afrazi A, Richardson W, Beer-Stolz D, Ma C, Prindle T, Grant Z, Branca M, Ozolek J, Hackam D
J Immunol, 2013-03-01;190(7):3541-51.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Generation of a germ cell-specific mouse transgenic CHERRY reporter, Sohlh1-mCherryFlag
Authors: Hitomi Suzuki, Christina Tenenhaus Dann, Aleksandar Rajkovic
genesis
-
N-cadherin+ HSCs in fetal liver exhibit higher long-term bone marrow reconstitution activity than N-cadherin- HSCs.
Biochem Biophys Res Commun, 2012-10-22;428(3):354-9.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC-Fr -
Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli.
Authors: Betterman K, Paquet-Fifield S, Asselin-Labat M, Visvader J, Butler L, Stacker S, Achen M, Harvey N
Am J Pathol, 2012-10-11;181(6):2225-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Neural crest contribution to lingual mesenchyme, epithelium and developing taste papillae and taste buds
Authors: Hong-Xiang Liu, Yoshihiro Komatsu, Yuji Mishina, Charlotte M. Mistretta
Developmental Biology
-
Epithelial cell-intrinsic notch signaling plays an essential role in the maintenance of gut immune homeostasis.
Authors: Obata Y, Takahashi D, Ebisawa M, Kakiguchi K, Yonemura S, Jinnohara T, Kanaya T, Fujimura Y, Ohmae M, Hase K, Ohno H
J. Immunol., 2012-01-25;188(5):2427-36.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Separate and distinctive roles for Wnt5a in tongue, lingual tissue and taste papilla development
Authors: Hong-Xiang Liu, Ann S. Grosse, Ken Iwatsuki, Yuji Mishina, Deborah L. Gumucio, Charlotte M. Mistretta
Developmental Biology
Species: Mouse, Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Noninvasive assessment of antenatal hydronephrosis in mice reveals a critical role for Robo2 in maintaining anti-reflux mechanism.
Authors: Wang H, Li Q, Liu J
PLoS ONE, 2011-09-20;6(9):e24763.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors.
Authors: Nakahara T, Norberg SM, Shalinsky DR, Hu-Lowe DD, McDonald DM
Cancer Res., 2006-02-01;66(3):1434-45.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons.
Authors: Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR
Development, 2005-07-27;132(17):3847-57.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice.
Authors: Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA
Kidney Int., 2005-06-01;67(6):2221-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr, IHC-P -
Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis.
Authors: Egan CE, Sodhi CP, Good M et al.
J. Clin. Invest.
-
Telophase correction refines division orientation in stratified epithelia
Authors: KJ Lough, KM Byrd, CP Descovich, DC Spitzer, AJ Bergman, GM Beaudoin, LF Reichardt, SE Williams
Elife, 2019-12-13;8(0):.
-
Dental Epithelial Stem Cells as a Source for Mammary Gland Regeneration and Milk Producing Cells In Vivo
Authors: L Jimenez-Ro, P Pagella, H Harada, TA Mitsiadis
Cells, 2019-10-22;8(10):.
-
Recapitulating kidney development in vitro by priming and differentiating mouse embryonic stem cells in monolayers.
Authors: Chow T, Wong F T M et al.
NPJ Regen Med
-
Retinoic Acid Improves Incidence and Severity of Necrotizing Enterocolitis by Lymphocyte Balance Restitution and Repopulation of LGR5+ Intestinal Stem Cells.
Authors: Nino DF, Sodhi CP, Egan CE et al.
Shock
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsIsotype Controls
Reconstitution Buffers
Secondary Antibodies
Reviews for Human/Mouse E-Cadherin Antibody
Average Rating: 4.5 (Based on 11 Reviews)
Have you used Human/Mouse E-Cadherin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
HepG2 40 ug cell lysate loaded. Aspecific bands appeared at 55 kDa