Human PDGF R alpha Antibody Summary
Gln24-Glu524
Accession # P16234
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Cell Proliferation Induced by PDGF‑AA and Neutralization by Human PDGF R alpha Antibody. Recombinant Human PDGF-AA (Catalog # 221-AA) stimulates proliferation in the WS-1 human fetal skin fibroblast cell line in a dose-dependent manner (orange line). Proliferation elicited by Recombinant Human PDGF-AA (10 ng/mL) is neutralized (green line) by increasing concentrations of Goat Anti-Human PDGF Ra Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-307-NA). The ND50 is typically 1-6 µg/mL.
 View Larger
View Larger
PDGF R alpha in Human Breast Cancer Tissue. PDGF Ra was detected in immersion fixed paraffin-embedded sections of human breast cancer tissue using Goat Anti-Human PDGF Ra Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-307-NA) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Lower panel shows a lack of labeling if primary antibodies are omitted and tissue is stained only with secondary antibody followed by incubation with detection reagents. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
PDGF R alpha in Human Ovary. PDGF Ra was detected in immersion fixed paraffin-embedded sections of human ovarian array using Goat Anti-Human PDGF Ra Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-307-NA) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
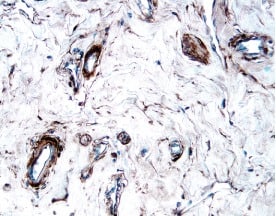
PDGF R alpha in Human Osteosarcoma. PDGF Ra was detected in immersion fixed paraffin-embedded sections of human osteosarcoma using Goat Anti-Human PDGF Ra Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-307-NA) at 3 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific staining was localized to plasma membranes. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal human corneal sections. (A–C) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) CD34‐positive stromal cells are orderly arranged and parallel to the corneal surface. (B and C) At higher magnification, the CD34‐positive stromal cells appear as spindle‐shaped cells with a small oval body and typically two long and thin moniliform cell processes characterized by the alternation of slender segments (arrows) and knobs/dilations (arrowheads) along their length. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. Colocalization of CD34 and PDGFR alpha in stromal cells gives rise to yellow staining either in the anterior corneal stroma (D–F) or in the deeper corneal stromal layer (G–I). CD34+/PDGFR alpha + stromal cells display cell morphologies very evocative for telocytes: a small cell body with very long prolongations (telopodes) characterized by a moniliform silhouette with the alternation of podoms (arrowheads) and podomers (arrows). (J–L) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. Either in the subepithelial corneal stroma or in the deeper stromal layer, numerous CD34‐positive stromal cells coexpress c‐kit. Inset: Higher magnification view of CD34+/c‐kit+ corneal stromal cells. Scale bar: 50 μm (A, D–F and J–L), 25 μm (B, C and G–I). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal and keratoconic human corneal sections. (A and B) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) In control normal corneas, CD34‐positive stromal cells displaying morphological features of telocytes are orderly arranged and parallel to the corneal surface. (B) In keratoconus, a patchy loss of CD34‐positive stromal cells is mostly evident in the anterior corneal stroma. Insets: Higher magnification views of CD34‐positive corneal stromal cells. (C) Results of quantitative analysis of CD34‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. (D–F) In control normal corneas, CD34+/PDGFR alpha + stromal cells (telocytes) are orderly distributed throughout the stromal compartment. (G–I) In keratoconic corneas, a patchy loss of CD34+/PDGFR alpha + stromal cells (telocytes) is mainly evident in the subepithelial stroma. (J and K) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. (J) In control normal corneas, numerous CD34+/c‐kit+ stromal cells (telocytes) are present throughout the stromal layer. (K) In keratoconic corneas, the CD34+/c‐kit+ stromal cell subpopulation is almost completely lost. (L) Results of quantitative analysis of c‐kit‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. TC: telocytes; hpf: high‐power field. Scale bar: 100 μm (A and B), 50 μm (D–K). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal and keratoconic human corneal sections. (A and B) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) In control normal corneas, CD34‐positive stromal cells displaying morphological features of telocytes are orderly arranged and parallel to the corneal surface. (B) In keratoconus, a patchy loss of CD34‐positive stromal cells is mostly evident in the anterior corneal stroma. Insets: Higher magnification views of CD34‐positive corneal stromal cells. (C) Results of quantitative analysis of CD34‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. (D–F) In control normal corneas, CD34+/PDGFR alpha + stromal cells (telocytes) are orderly distributed throughout the stromal compartment. (G–I) In keratoconic corneas, a patchy loss of CD34+/PDGFR alpha + stromal cells (telocytes) is mainly evident in the subepithelial stroma. (J and K) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. (J) In control normal corneas, numerous CD34+/c‐kit+ stromal cells (telocytes) are present throughout the stromal layer. (K) In keratoconic corneas, the CD34+/c‐kit+ stromal cell subpopulation is almost completely lost. (L) Results of quantitative analysis of c‐kit‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. TC: telocytes; hpf: high‐power field. Scale bar: 100 μm (A and B), 50 μm (D–K). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal and keratoconic human corneal sections. (A and B) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) In control normal corneas, CD34‐positive stromal cells displaying morphological features of telocytes are orderly arranged and parallel to the corneal surface. (B) In keratoconus, a patchy loss of CD34‐positive stromal cells is mostly evident in the anterior corneal stroma. Insets: Higher magnification views of CD34‐positive corneal stromal cells. (C) Results of quantitative analysis of CD34‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. (D–F) In control normal corneas, CD34+/PDGFR alpha + stromal cells (telocytes) are orderly distributed throughout the stromal compartment. (G–I) In keratoconic corneas, a patchy loss of CD34+/PDGFR alpha + stromal cells (telocytes) is mainly evident in the subepithelial stroma. (J and K) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. (J) In control normal corneas, numerous CD34+/c‐kit+ stromal cells (telocytes) are present throughout the stromal layer. (K) In keratoconic corneas, the CD34+/c‐kit+ stromal cell subpopulation is almost completely lost. (L) Results of quantitative analysis of c‐kit‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. TC: telocytes; hpf: high‐power field. Scale bar: 100 μm (A and B), 50 μm (D–K). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal human corneal sections. (A–C) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) CD34‐positive stromal cells are orderly arranged and parallel to the corneal surface. (B and C) At higher magnification, the CD34‐positive stromal cells appear as spindle‐shaped cells with a small oval body and typically two long and thin moniliform cell processes characterized by the alternation of slender segments (arrows) and knobs/dilations (arrowheads) along their length. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. Colocalization of CD34 and PDGFR alpha in stromal cells gives rise to yellow staining either in the anterior corneal stroma (D–F) or in the deeper corneal stromal layer (G–I). CD34+/PDGFR alpha + stromal cells display cell morphologies very evocative for telocytes: a small cell body with very long prolongations (telopodes) characterized by a moniliform silhouette with the alternation of podoms (arrowheads) and podomers (arrows). (J–L) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. Either in the subepithelial corneal stroma or in the deeper stromal layer, numerous CD34‐positive stromal cells coexpress c‐kit. Inset: Higher magnification view of CD34+/c‐kit+ corneal stromal cells. Scale bar: 50 μm (A, D–F and J–L), 25 μm (B, C and G–I). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal and keratoconic human corneal sections. (A and B) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) In control normal corneas, CD34‐positive stromal cells displaying morphological features of telocytes are orderly arranged and parallel to the corneal surface. (B) In keratoconus, a patchy loss of CD34‐positive stromal cells is mostly evident in the anterior corneal stroma. Insets: Higher magnification views of CD34‐positive corneal stromal cells. (C) Results of quantitative analysis of CD34‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. (D–F) In control normal corneas, CD34+/PDGFR alpha + stromal cells (telocytes) are orderly distributed throughout the stromal compartment. (G–I) In keratoconic corneas, a patchy loss of CD34+/PDGFR alpha + stromal cells (telocytes) is mainly evident in the subepithelial stroma. (J and K) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. (J) In control normal corneas, numerous CD34+/c‐kit+ stromal cells (telocytes) are present throughout the stromal layer. (K) In keratoconic corneas, the CD34+/c‐kit+ stromal cell subpopulation is almost completely lost. (L) Results of quantitative analysis of c‐kit‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. TC: telocytes; hpf: high‐power field. Scale bar: 100 μm (A and B), 50 μm (D–K). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal and keratoconic human corneal sections. (A and B) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) In control normal corneas, CD34‐positive stromal cells displaying morphological features of telocytes are orderly arranged and parallel to the corneal surface. (B) In keratoconus, a patchy loss of CD34‐positive stromal cells is mostly evident in the anterior corneal stroma. Insets: Higher magnification views of CD34‐positive corneal stromal cells. (C) Results of quantitative analysis of CD34‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. (D–F) In control normal corneas, CD34+/PDGFR alpha + stromal cells (telocytes) are orderly distributed throughout the stromal compartment. (G–I) In keratoconic corneas, a patchy loss of CD34+/PDGFR alpha + stromal cells (telocytes) is mainly evident in the subepithelial stroma. (J and K) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. (J) In control normal corneas, numerous CD34+/c‐kit+ stromal cells (telocytes) are present throughout the stromal layer. (K) In keratoconic corneas, the CD34+/c‐kit+ stromal cell subpopulation is almost completely lost. (L) Results of quantitative analysis of c‐kit‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. TC: telocytes; hpf: high‐power field. Scale bar: 100 μm (A and B), 50 μm (D–K). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal human corneal sections. (A–C) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) CD34‐positive stromal cells are orderly arranged and parallel to the corneal surface. (B and C) At higher magnification, the CD34‐positive stromal cells appear as spindle‐shaped cells with a small oval body and typically two long and thin moniliform cell processes characterized by the alternation of slender segments (arrows) and knobs/dilations (arrowheads) along their length. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. Colocalization of CD34 and PDGFR alpha in stromal cells gives rise to yellow staining either in the anterior corneal stroma (D–F) or in the deeper corneal stromal layer (G–I). CD34+/PDGFR alpha + stromal cells display cell morphologies very evocative for telocytes: a small cell body with very long prolongations (telopodes) characterized by a moniliform silhouette with the alternation of podoms (arrowheads) and podomers (arrows). (J–L) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. Either in the subepithelial corneal stroma or in the deeper stromal layer, numerous CD34‐positive stromal cells coexpress c‐kit. Inset: Higher magnification view of CD34+/c‐kit+ corneal stromal cells. Scale bar: 50 μm (A, D–F and J–L), 25 μm (B, C and G–I). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal human corneal sections. (A–C) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) CD34‐positive stromal cells are orderly arranged and parallel to the corneal surface. (B and C) At higher magnification, the CD34‐positive stromal cells appear as spindle‐shaped cells with a small oval body and typically two long and thin moniliform cell processes characterized by the alternation of slender segments (arrows) and knobs/dilations (arrowheads) along their length. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. Colocalization of CD34 and PDGFR alpha in stromal cells gives rise to yellow staining either in the anterior corneal stroma (D–F) or in the deeper corneal stromal layer (G–I). CD34+/PDGFR alpha + stromal cells display cell morphologies very evocative for telocytes: a small cell body with very long prolongations (telopodes) characterized by a moniliform silhouette with the alternation of podoms (arrowheads) and podomers (arrows). (J–L) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. Either in the subepithelial corneal stroma or in the deeper stromal layer, numerous CD34‐positive stromal cells coexpress c‐kit. Inset: Higher magnification view of CD34+/c‐kit+ corneal stromal cells. Scale bar: 50 μm (A, D–F and J–L), 25 μm (B, C and G–I). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal human corneal sections. (A–C) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) CD34‐positive stromal cells are orderly arranged and parallel to the corneal surface. (B and C) At higher magnification, the CD34‐positive stromal cells appear as spindle‐shaped cells with a small oval body and typically two long and thin moniliform cell processes characterized by the alternation of slender segments (arrows) and knobs/dilations (arrowheads) along their length. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. Colocalization of CD34 and PDGFR alpha in stromal cells gives rise to yellow staining either in the anterior corneal stroma (D–F) or in the deeper corneal stromal layer (G–I). CD34+/PDGFR alpha + stromal cells display cell morphologies very evocative for telocytes: a small cell body with very long prolongations (telopodes) characterized by a moniliform silhouette with the alternation of podoms (arrowheads) and podomers (arrows). (J–L) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. Either in the subepithelial corneal stroma or in the deeper stromal layer, numerous CD34‐positive stromal cells coexpress c‐kit. Inset: Higher magnification view of CD34+/c‐kit+ corneal stromal cells. Scale bar: 50 μm (A, D–F and J–L), 25 μm (B, C and G–I). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal human corneal sections. (A–C) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) CD34‐positive stromal cells are orderly arranged and parallel to the corneal surface. (B and C) At higher magnification, the CD34‐positive stromal cells appear as spindle‐shaped cells with a small oval body and typically two long and thin moniliform cell processes characterized by the alternation of slender segments (arrows) and knobs/dilations (arrowheads) along their length. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. Colocalization of CD34 and PDGFR alpha in stromal cells gives rise to yellow staining either in the anterior corneal stroma (D–F) or in the deeper corneal stromal layer (G–I). CD34+/PDGFR alpha + stromal cells display cell morphologies very evocative for telocytes: a small cell body with very long prolongations (telopodes) characterized by a moniliform silhouette with the alternation of podoms (arrowheads) and podomers (arrows). (J–L) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. Either in the subepithelial corneal stroma or in the deeper stromal layer, numerous CD34‐positive stromal cells coexpress c‐kit. Inset: Higher magnification view of CD34+/c‐kit+ corneal stromal cells. Scale bar: 50 μm (A, D–F and J–L), 25 μm (B, C and G–I). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PDGFR alpha by Immunocytochemistry/Immunofluorescence Representative light and fluorescence microscopy photomicrographs of normal and keratoconic human corneal sections. (A and B) CD34 immunoperoxidase‐based immunohistochemistry with haematoxylin counterstain. (A) In control normal corneas, CD34‐positive stromal cells displaying morphological features of telocytes are orderly arranged and parallel to the corneal surface. (B) In keratoconus, a patchy loss of CD34‐positive stromal cells is mostly evident in the anterior corneal stroma. Insets: Higher magnification views of CD34‐positive corneal stromal cells. (C) Results of quantitative analysis of CD34‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. (D–I) Double immunofluorescence labelling for CD34 (red) and PDGFR alpha (green) with DAPI (blue) counterstain for nuclei. (D–F) In control normal corneas, CD34+/PDGFR alpha + stromal cells (telocytes) are orderly distributed throughout the stromal compartment. (G–I) In keratoconic corneas, a patchy loss of CD34+/PDGFR alpha + stromal cells (telocytes) is mainly evident in the subepithelial stroma. (J and K) Double immunofluorescence labelling for CD34 (red) and c‐kit (green) with DAPI (blue) counterstain for nuclei. (J) In control normal corneas, numerous CD34+/c‐kit+ stromal cells (telocytes) are present throughout the stromal layer. (K) In keratoconic corneas, the CD34+/c‐kit+ stromal cell subpopulation is almost completely lost. (L) Results of quantitative analysis of c‐kit‐positive telocyte counts per high‐power field in the corneal stroma of healthy controls (n = 6) and patients with keratoconus (n = 6). Data are mean ± S.D. *P < 0.001 versus control. TC: telocytes; hpf: high‐power field. Scale bar: 100 μm (A and B), 50 μm (D–K). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28714595), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: PDGF R alpha
PDGF is a major serum mitogen that can exist as a homo- or heterodimeric protein consisting of disulfide-linked PDGF-A and PDGF-B chains. The PDGF‑AA, PDGF‑BB, and PDGF‑AB isoforms have been shown to bind to two distinct cell surface PDGF receptors with different affinities. Whereas PDGF R alpha binds all three PDGF isoforms with high affinity, PDGF R beta binds PDGF-BB and -AB, but not PDGF-AA. Both PDGF R alpha and PDGF R beta are members of the class III subfamily of receptor tyrosine kinases (RTK) that also includes the receptors for M-CSF, SCF, and Flt-3 ligand. All class III RTKs are characterized by the presence of five immunoglobulin-like domains in their extracellular region and a split kinase domain in their intracellular region. PDGF binding induces receptor homo-and heterodimerization and signal transduction. The expression of the alpha and beta receptors is independently regulated in various cell types. Only PDGF R alpha is expressed in oligodendrocyte progenitor cells, mesothelial cell, and liver endothelial cells. Soluble PDGF R alpha has been detected in cell conditioned medium and human plasma. Recombinant soluble PDGF R alpha binds PDGF with high affinity and is a potent PDGF antagonist (1).
- Heldin, C.H. and L. Claesson-Welsh (1994) Guidebook to Cytokines and Their Receptors, Nicola, N.A. (ed) Oxford University Press, New York, NY p. 202.
Product Datasheets
Citations for Human PDGF R alpha Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
57
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease.
Authors: Kinchen J, Chen HH, Parikh K et al.
Cell
-
PDGF Receptor-alpha Does Not Promote HCMV Entry into Epithelial and Endothelial Cells but Increased Quantities Stimulate Entry by an Abnormal Pathway
Authors: Vanarsdall AL, Wisner TW, Lei H et al.
PLoS Pathog
-
Platelet-derived growth factor receptor-alpha is essential for cardiac fibroblast survival
Authors: Malina J. Ivey, Jill T. Kuwabara, Kara L. Riggsbee, Michelle D. Tallquist
American Journal of Physiology-Heart and Circulatory Physiology
-
High-throughput screening for myelination promoting compounds using human stem cell-derived oligodendrocyte progenitor cells
Authors: Weifeng Li, Cynthia Berlinicke, Yinyin Huang, Stefanie Giera, Anna G. McGrath, Weixiang Fang et al.
iScience
-
A human autoimmune organoid model reveals IL-7 function in coeliac disease
Authors: Santos, AJM;van Unen, V;Lin, Z;Chirieleison, SM;Ha, N;Batish, A;Chan, JE;Cedano, J;Zhang, ET;Mu, Q;Guh-Siesel, A;Tomaske, M;Colburg, D;Varma, S;Choi, SS;Christophersen, A;Baghdasaryan, A;Yost, KE;Karlsson, K;Ha, A;Li, J;Dai, H;Sellers, ZM;Chang, HY;Dunn, JCY;Zhang, BM;Mellins, ED;Sollid, LM;Fernandez-Becker, NQ;Davis, MM;Kuo, CJ;
Nature
Species: Human
Sample Types: Organoid
Applications: Immunohistochemistry -
Harnessing developmental dynamics of spinal cord extracellular matrix improves regenerative potential of spinal cord organoids
Authors: Sun, Z;Chen, Z;Yin, M;Wu, X;Guo, B;Cheng, X;Quan, R;Sun, Y;Zhang, Q;Fan, Y;Jin, C;Yin, Y;Hou, X;Liu, W;Shu, M;Xue, X;Shi, Y;Chen, B;Xiao, Z;Dai, J;Zhao, Y;
Cell stem cell
Species: Rabbit, Rat
Sample Types: Organoid
Applications: Immunohistochemistry -
Patterning and folding of intestinal villi by active mesenchymal dewetting
Authors: Huycke, TR;Miyazaki, H;Häkkinen, TJ;Srivastava, V;Barruet, E;McGinnis, CS;Kalantari, A;Cornwall-Scoones, J;Vaka, D;Zhu, Q;Jo, H;DeGrado, WF;Thomson, M;Garikipati, K;Boffelli, D;Klein, OD;Gartner, ZJ;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Organoids
Applications: IHC -
Metformin promotes Schwann cell remyelination, preserves neural tissue and improves functional recovery after spinal cord injury
Authors: Huang, Z;Lin, J;Jiang, H;Lin, W;Huang, Z;Chen, J;Xiao, W;Lin, Q;Wang, J;Wen, S;Zhu, Q;Liu, J;
Neuropeptides
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Non-myogenic mesenchymal cells contribute to muscle degeneration in facioscapulohumeral muscular dystrophy patients
Authors: L Di Pietro, F Giacalone, E Ragozzino, V Saccone, F Tiberio, M De Bardi, M Picozza, G Borsellino, W Lattanzi, E Guadagni, S Bortolani, G Tasca, E Ricci, O Parolini
Cell Death & Disease, 2022-09-16;13(9):793.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Oviductal Oxygen Homeostasis in Patients with Uterine Myoma: Correlation between Hypoxia and Telocytes
Authors: A Wrona, V Aleksandro, T Bereza, P Basta, A Gil, M Ulatowska-, M Mazur-Lask, K Pity?ski, K Gil
International Journal of Molecular Sciences, 2022-05-31;23(11):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Morphologic evidence of telocytes in human thyroid stromal tissue
Authors: I Rosa, L Ibba-Manne, D Guasti, G Perigli, MS Faussone-P, M Manetti
Journal of Cellular and Molecular Medicine, 2022-03-20;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Developmental Origins of Human Cortical Oligodendrocytes and Astrocytes
Authors: Lin Yang, Zhenmeiyu Li, Guoping Liu, Xiaosu Li, Zhengang Yang
Neuroscience Bulletin
-
Deciphering the spatial-temporal transcriptional landscape of human hypothalamus development
Authors: X Zhou, Y Lu, F Zhao, J Dong, W Ma, S Zhong, M Wang, B Wang, Y Zhao, Y Shi, Q Ma, T Lu, J Zhang, X Wang, Q Wu
Cell Stem Cell, 2021-12-07;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Human skeletal muscle CD90+ fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients
Authors: Jean Farup, Jesper Just, Frank de Paoli, Lin Lin, Jonas Brorson Jensen, Tine Billeskov et al.
Cell Metabolism
Species: Human
Sample Types: Tissue Homogenates, Whole Cells
Applications: Western Blot, Immunocytochemistry -
IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies
Authors: M Friedrich, M Pohin, MA Jackson, I Korsunsky, SJ Bullers, K Rue-Albrec, Z Christofor, D Sathananth, T Thomas, R Ravindran, R Tandon, RS Peres, H Sharpe, K Wei, GFM Watts, EH Mann, A Geremia, M Attar, Oxford IBD, Roche Fibr, S McCuaig, L Thomas, E Collantes, HH Uhlig, SN Sansom, A Easton, S Raychaudhu, SP Travis, FM Powrie
Nature Medicine, 2021-10-21;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Oviductal Telocytes in Patients with Uterine Myoma
Authors: V Aleksandro, A Wrona, T Bereza, K Pity?ski, K Gil
Biomedicines, 2021-08-20;9(8):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer's disease
Authors: S Morabito, E Miyoshi, N Michael, S Shahin, AC Martini, E Head, J Silva, K Leavy, M Perez-Rose, V Swarup
Nature Genetics, 2021-07-08;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
CD109-GP130 interaction drives glioblastoma stem cell plasticity and chemoresistance through STAT3 activity
Authors: P Filppu, JT Ramanathan, KJ Granberg, E Gucciardo, H Haapasalo, K Lehti, M Nykter, V Le Joncour, P Laakkonen
JCI Insight, 2021-05-10;6(9):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Heterogeneity of glial progenitor cells during the neurogenesis-to-gliogenesis switch in the developing human cerebral cortex
Authors: Y Fu, M Yang, H Yu, Y Wang, X Wu, J Yong, Y Mao, Y Cui, X Fan, L Wen, J Qiao, F Tang
Cell Reports, 2021-03-02;34(9):108788.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Histone H3.3G34-Mutant Interneuron Progenitors Co-opt PDGFRA for Gliomagenesis
Authors: Carol C.L. Chen, Shriya Deshmukh, Selin Jessa, Djihad Hadjadj, Véronique Lisi, Augusto Faria Andrade et al.
Cell
-
Origins and Proliferative States of Human Oligodendrocyte Precursor Cells
Authors: Wei Huang, Aparna Bhaduri, Dmitry Velmeshev, Shaohui Wang, Li Wang, Catherine A. Rottkamp et al.
Cell
-
Blood flow-restricted resistance exercise alters the surface profile, miRNA cargo and functional impact of circulating extracellular vesicles
Authors: J Just, Y Yan, J Farup, P Sieljacks, M Sloth, M Venø, T Gu, FV de Paoli, JR Nyengaard, R Bæk, MM Jørgensen, J Kjems, K Vissing, KR Drasbek
Sci Rep, 2020-04-03;10(1):5835.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Differential Expression of PDGF Receptor-alpha in Human Placental Trophoblasts Leads to Different Entry Pathways by Human Cytomegalovirus Strains
Authors: Zin Naing, Stuart T. Hamilton, Wendy J. van Zuylen, Gillian M. Scott, William D. Rawlinson
Scientific Reports
-
Patch repair of deep wounds by mobilized fascia
Authors: D Correa-Gal, D Jiang, S Christ, P Ramesh, H Ye, J Wannemache, S Kalgudde G, Q Yu, M Aichler, A Walch, U Mirastschi, T Volz, Y Rinkevich
Nature, 2019-11-27;0(0):.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC -
Identification of PDGFR alpha + cells in uterine fibroids – link between angiogenesis and uterine telocytes
Authors: Veronika Aleksandrovych, Tomasz Bereza, Magdalena Ulatowska-Białas, Artur Pasternak, Jerzy A. A. Walocha, Kazimierz Pityński et al.
Archives of Medical Science
-
An Immunohistochemical Study of Gastric Mucosa and Critical Review Indicate That the Subepithelial Telocytes Are Prelymphatic Endothelial Cells
Authors: Oana D. Toader, Mugurel C. Rusu, Laurenţiu Mogoantă, Sorin Hostiuc, Adelina Maria Jianu, Adrian Cosmin Ilie
Medicina (Kaunas)
-
The Autonomic Innervation and Uterine Telocyte Interplay in Leiomyoma Formation
Authors: Veronika Aleksandrovych, Magdalena Kurnik-Łucka, Tomasz Bereza, Magdalena Białas, Artur Pasternak, Dragos Cretoiu et al.
Cell Transplantation
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Anterior Cruciate Ligament Tear Promotes Skeletal Muscle Myostatin Expression, Fibrogenic Cell Expansion, and a Decline in Muscle Quality
Authors: Bailey D. Peck, Camille R. Brightwell, Darren L. Johnson, Mary Lloyd Ireland, Brian Noehren, Christopher S. Fry
The American Journal of Sports Medicine
-
Induction of nuclear protein-1 by thyroid hormone enhances platelet-derived growth factor A mediated angiogenesis in liver cancer
Authors: CY Chen, SM Wu, YH Lin, HC Chi, SL Lin, CT Yeh, WY Chuang, KH Lin
Theranostics, 2019-04-13;9(8):2361-2379.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Telocytes constitute a widespread interstitial meshwork in the lamina propria and underlying striated muscle of human tongue
Authors: Irene Rosa, Cecilia Taverna, Luca Novelli, Mirca Marini, Lidia Ibba-Manneschi, Mirko Manetti
Scientific Reports
-
Skeletal muscle fibrosis is associated with decreased muscle inflammation and weakness in patients with chronic kidney disease
Authors: Matthew K. Abramowitz, William Paredes, Kehao Zhang, Camille R. Brightwell, Julia N. Newsom, Hyok-Joon Kwon et al.
American Journal of Physiology-Renal Physiology
-
Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination
Authors: Greg J. Duncan, Sohrab B. Manesh, Brett J. Hilton, Peggy Assinck, Jie Liu, Aaron Moulson et al.
Nature Communications
-
Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations
Authors: Christina Philippeos, Stephanie B. Telerman, Bénédicte Oulès, Angela O. Pisco, Tanya J. Shaw, Raul Elgueta et al.
Journal of Investigative Dermatology
-
Morphological evidence of telocytes in human synovium
Authors: I Rosa, M Marini, D Guasti, L Ibba-Manne, M Manetti
Sci Rep, 2018-02-26;8(1):3581.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Transcriptome analysis of PDGFR?+ cells identifies T-type Ca2+ channel CACNA1G as a new pathological marker for PDGFR?+ cell hyperplasia
Authors: SE Ha, MY Lee, M Kurahashi, L Wei, BG Jorgensen, C Park, PJ Park, D Redelman, KC Sasse, LS Becker, KM Sanders, S Ro
PLoS ONE, 2017-08-14;12(8):e0182265.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr -
Adaptive changes of telocytes in the urinary bladder of patients affected by neurogenic detrusor overactivity
Authors: C Traini, MS Fausssone-, D Guasti, G Del Popolo, J Frizzi, S Serni, MG Vannucchi
J. Cell. Mol. Med., 2017-08-07;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Telocytes in normal and keratoconic human cornea: an immunohistochemical and transmission electron microscopy study
Authors: M Marini, R Mencucci, I Rosa, E Favuzza, D Guasti, L Ibba-Manne, M Manetti
J. Cell. Mol. Med., 2017-07-17;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Non-glycanated Decorin Is a Drug Target on Human Adipose Stromal Cells
Authors: AC Daquinag, A Dadbin, B Snyder, X Wang, AA Sahin, NT Ueno, MG Kolonin
Mol Ther Oncolytics, 2017-05-17;6(0):1-9.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
ACL injury reduces satellite cell abundance and promotes fibrogenic cell expansion within skeletal muscle
Authors: Christopher S Fry
J. Orthop. Res, 2017-01-15;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Telocytes in minor salivary glands of primary Sjögren's syndrome: association with the extent of inflammation and ectopic lymphoid neogenesis
Authors: Alessia Alunno, Lidia Ibba-Manneschi, Onelia Bistoni, Irene Rosa, Sara Caterbi, Roberto Gerli et al.
Journal of Cellular and Molecular Medicine
-
Telocytes subtypes in human urinary bladder
Authors: Maria‐Giuliana Vannucchi, Chiara Traini, Daniele Guasti, Del Popolo Del Popolo, Maria‐Simonetta Faussone‐Pellegrini
Journal of Cellular and Molecular Medicine
-
Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle.
Authors: Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, Morita M, Yamaguchi A, Yamada H, Nishino I, Hamada Y, Tsuchida K
Cell Death Dis, 2014-04-17;5(0):e1186.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr -
Isoflurane-induced Apoptosis of Neurons and Oligodendrocytes in the Fetal Rhesus Macaque Brain
Authors: Catherine E. Creeley, Krikor T. Dikranian, Gregory A. Dissen, Stephen A. Back, John W. Olney, Ansgar M. Brambrink
Anesthesiology
-
Alcohol-induced apoptosis of oligodendrocytes in the fetal macaque brain
Authors: Catherine E Creeley, Krikor T Dikranian, Stephen A Johnson, Nuri B Farber, John W Olney
Acta Neuropathologica Communications
-
Telocytes in Crohn's disease.
Authors: Milia A, Ruffo M, Manetti M, Rosa I, Conte D, Fazi M, Messerini L, Ibba-Manneschi L
J Cell Mol Med, 2013-11-19;17(12):1525-36.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Telocytes express PDGFR alpha in the human gastrointestinal tract
Authors: Maria‐Giuliana Vannucchi, Chiara Traini, Mirko Manetti, Lidia Ibba‐Manneschi, Maria‐Simonetta Faussone‐Pellegrini
Journal of Cellular and Molecular Medicine
-
Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain
Authors: Ansgar M. Brambrink, Stephen A. Back, Art Riddle, Xi Gong, Matthew D. Moravec, Gregory A. Dissen et al.
Annals of Neurology
-
Arhgef15 promotes retinal angiogenesis by mediating VEGF-induced Cdc42 activation and potentiating RhoJ inactivation in endothelial cells.
Authors: Kusuhara, Sentaro, Fukushima, Yoko, Fukuhara, Shigetom, Jakt, Lars Mar, Okada, Mitsuhir, Shimizu, Yuri, Hata, Masayuki, Nishida, Kohji, Negi, Akira, Hirashima, Masanori, Mochizuki, Naoki, Nishikawa, Shin-Ich, Uemura, Akiyoshi
PLoS ONE, 2012-09-21;7(9):e45858.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Platelet-derived growth factor receptor alpha -positive cells in the tunica muscularis of human colon
Authors: Masaaki Kurahashi, Yasuko Nakano, Grant W. Hennig, Sean M. Ward, Kenton M. Sanders
Journal of Cellular and Molecular Medicine
-
Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection.
Authors: Soroceanu L, Akhavan A, Cobbs CS
Nature, 2008-08-13;455(7211):391-5.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Distinctions between fetal and adult human platelet-derived growth factor-responsive neural precursors.
Authors: Chojnacki A, Kelly JJ, Hader W, Weiss S
Ann. Neurol., 2008-08-01;64(2):127-42.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
Human bone marrow activates the Akt pathway in metastatic prostate cells through transactivation of the alpha-platelet-derived growth factor receptor.
Authors: Dolloff NG, Russell MR, Loizos N, Fatatis A
Cancer Res., 2007-01-15;67(2):555-62.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Heterodimerization of FGF-receptor 1 and PDGF-receptor-alpha: a novel mechanism underlying the inhibitory effect of PDGF-BB on FGF-2 in human cells.
Authors: Faraone D, Aguzzi MS, Ragone G, Russo K, Capogrossi MC, Facchiano A
Blood, 2005-12-01;107(5):1896-902.
Species: Human
Sample Types: Whole Cells
Applications: ICC, Immunoprecipitation -
Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas.
Authors: Shikada Y, Yonemitsu Y, Koga T, Onimaru M, Nakano T, Okano S, Sata S, Nakagawa K, Yoshino I, Maehara Y, Sueishi K
Cancer Res., 2005-08-15;65(16):7241-8.
Species: Human
Sample Types: Cell Lysates
Applications: Immunodepletion, Immunoprecipitation -
Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty.
Authors: Nili N, Cheema AN, Giordano FJ, Barolet AW, Babaei S, Hickey R, Eskandarian MR, Smeets M, Butany J, Pasterkamp G, Strauss BH
Am. J. Pathol., 2003-09-01;163(3):869-78.
Species: Rabbit
Sample Types: Cell Lysates
Applications: Western Blot -
Content and activity of cAMP response element-binding protein regulate platelet-derived growth factor receptor-alpha content in vascular smooth muscles.
Authors: Watson PA, Vinson C, Nesterova A, Reusch JE
Endocrinology, 2002-08-01;143(8):2922-9.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Improved epicardial cardiac fibroblast generation from iPSCs
Authors: Whitehead AJ, Hocker JD, Ren B, Engler AJ
Journal of molecular and cellular cardiology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsIsotype Controls
Reconstitution Buffers
Secondary Antibodies
Reviews for Human PDGF R alpha Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Human PDGF R alpha Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: