Achieve Robust, Consistent Immune Cell Cultures with R&D Systems Cytokines
Achieve Robust, Consistent Immune Cell Cultures with R&D Systems Cytokines
T Cells
T Cells
Cytokines for T Cell Culture and Differentiation
Cytokines for T Cell Culture and Differentiation
Cytokines for Differentiating CD4+ T Helper Cell Subsets
Cytokines for Differentiating CD4+ T Helper Cell Subsets
Cytokines for Differentiating CD8+ Cytotoxic T Cell Subsets
Cytokines for Differentiating CD8+ Cytotoxic T Cell Subsets
Natural Killer Cells
Natural Killer Cells
Cytokines for NK Cell Culture and Differentiation
Cytokines for NK Cell Culture and Differentiation
Monocytes/Macrophages
Monocytes/Macrophages
Cytokines for Monocyte/Macrophage Culture and Differentiation
Cytokines for Monocyte/Macrophage Culture and Differentiation
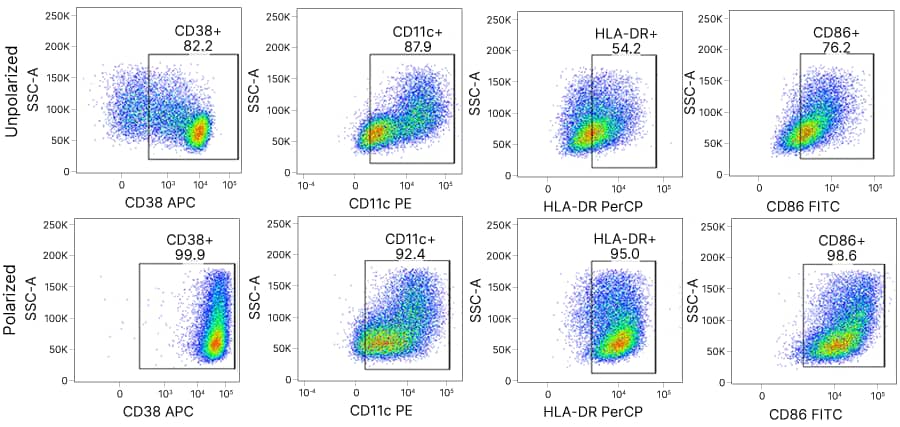
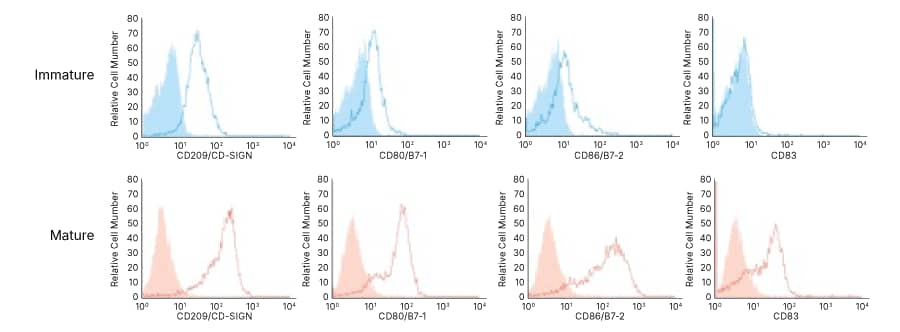
Dendritic Cells
Dendritic Cells
Cytokines for Dendritic Cell Culture, Activation, and Maturation
Cytokines for Dendritic Cell Culture, Activation, and Maturation
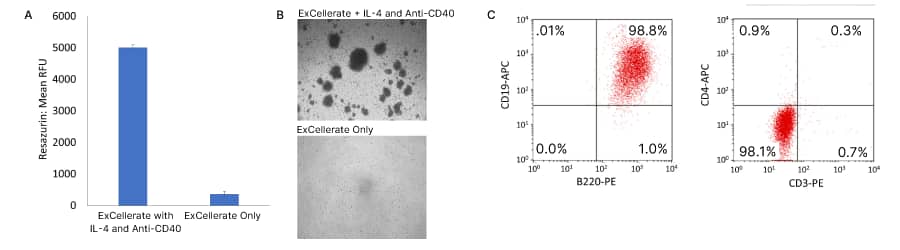
B Cells
B Cells
Cytokines for B Cell Culture and Differentiation
Cytokines for B Cell Culture and Differentiation
Antibodies for Immune Cell Activation and Differentiation
Antibodies for Immune Cell Activation and Differentiation
Additional Products to Improve Immune Cell Culture Consistency
Additional Products to Improve Immune Cell Culture Consistency
Immune Cell Culture Resources
Immune Cell Culture Resources
Featured Content
Featured Content
Featured Content

Explore the benefits of IL-2 Heat Stable Agonist Protein, which can withstand high temperatures and extended culture durations.
Featured Content

Learn about different cytokine families. This poster will serve as great reference tool and a colorful addition to your lab.
Featured Content

Receive competitive discounts on bulk quantities of a single item or multiple items in one order.
Featured Content

Proteins designed to meet your research needs: Novel proteins, specialized sizes & formulations, GMP conversion, and more.